Ta 183 Undergoes Radioactive Decay To Produce W 183 This Is An Example Of
Iupac Org Wp Content Uploads 15 02 Iptei Postprint Pdf
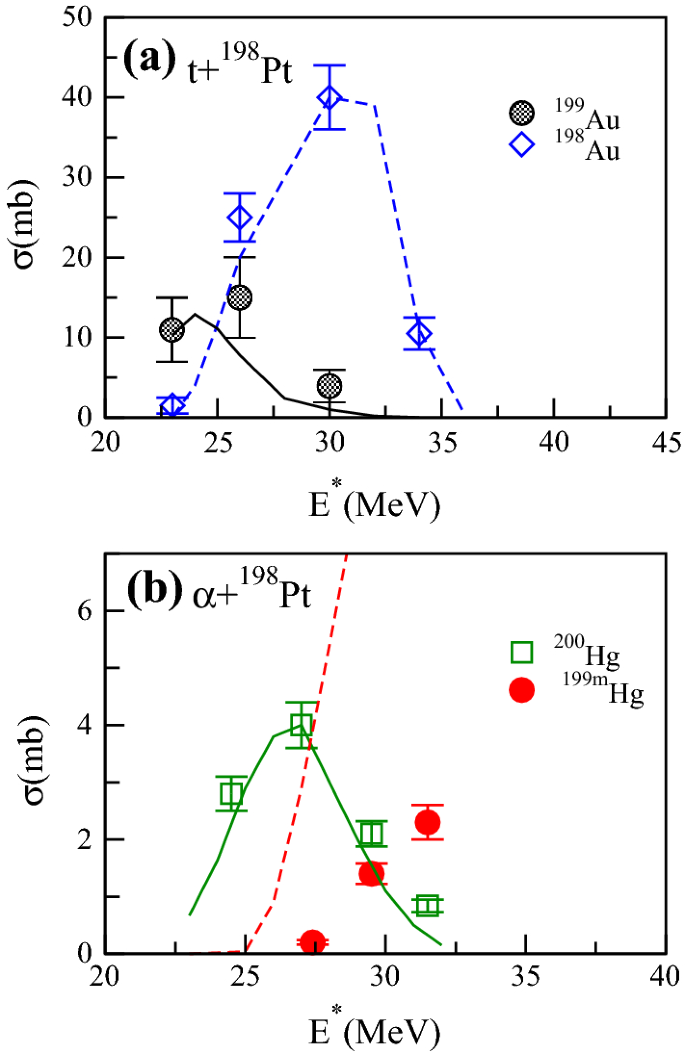
How To Extend The Chart Of Nuclides Springerlink
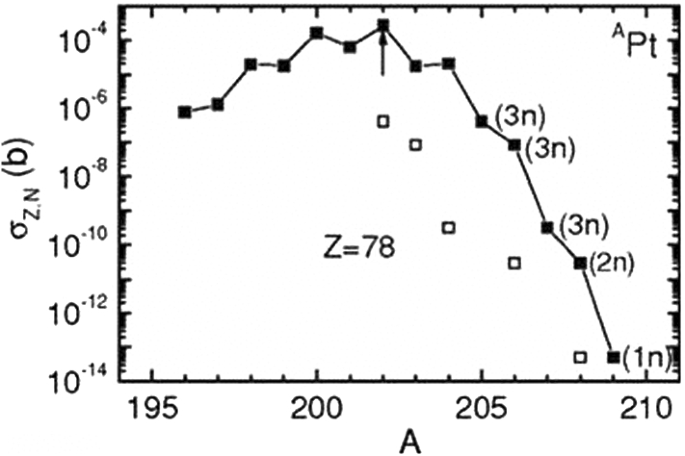
How To Extend The Chart Of Nuclides Springerlink
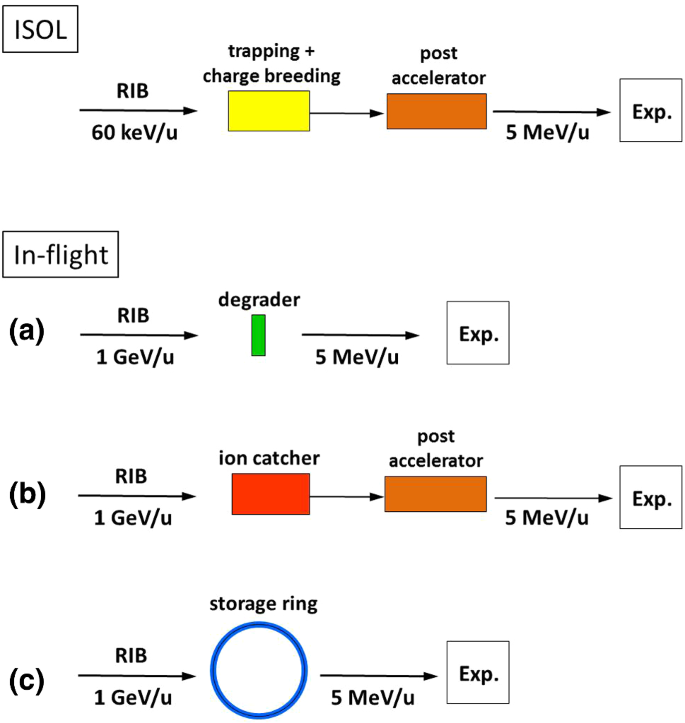
How To Extend The Chart Of Nuclides Springerlink
Www Energy Gov Sites Prod Files 13 10 F4 Qsr Radiationprotection Pdf

Isotopes Natural Miller Major Reference Works Wiley Online Library
The missing particles in the given radioactive decay processes are to be stated.
Ta 183 undergoes radioactive decay to produce w 183 this is an example of. Decay graphs and half lives article. The decay products within the chain are always radioactive. Radioactive Decay Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting radiation.
Thus it is the most commonly cited property of any radioisotope. Example (a) indicates that uranium-238 releases an alpha particle (i.e., a helium nucleus) to produce thorium-234. Iodine-131 is an example of a nuclide that undergoes β decay:.
One involves a change in mass number of the decaying nucleus, while others do not. I -10e+XorI -10β+XeI -10e+XorI -10β+Xe Beta decay, which can be thought of as the conversion of a neutron into a proton and a β particle, is observed in nuclides with a large n:p ratio. Letting N(t) stand for the number of radioactive nuclei in the sample at time t, the number of nuclei that decay in a time ∆t equals N(t) times the probability that one will decay in the time ∆t.
If a quantity of radioactive material produces one decay event per second, it has an activity of one Bq. Starting at a specific point in time, the material is monitored for decay events. Half life and decay rate.
(Consider the binding energy per nucleon.). This means they are unstable, and will eventually decay by emitting a particle, transforming the nucleus into another nucleus, or into a lower energy state. 6.1 LAW OF RADIOACTIVE DECAY The fundamental law of radioactive decay is based on the fact that the decay, i.e.
For example, ifHT is to represent the dose associated with a single year of exposure then the integration in Eq. This is the currently selected item. And a third was uncharged electromagnetic waves, γ rays.
Example of a radioactive decay chain from lead-212 (212Pb) to lead-8 (8Pb). For example, if radioactive decay isn't constant, then adjustments will have to be made for its use in dating materials, especially in the case of Carbon-14 dating. An example of such a process is:.
The correct response is c. (1) would range from t0to t0 + 1 years, where t0 is the year of interest. A chain of decays takes place until a stable nucleus is reached.
∫ =− ∫dt N dN λ ln N =−λt +c where c is an. Their nucleus breaks apart, undergoing nuclear decay. (Only a handful of nuclides with atomic numbers less than emit an -particle.)The product of -decay is easy to predict if we assume that both mass and charge are conserved in nuclear reactions.Alpha decay of the 238 U "parent" nuclide, for example, produces 234 Th as the "daughter" nuclide.
If radioactive decay began with 400,000 parent atoms, how many would be left after 3 half lives?. The third class of radioactive decay is gamma decay, in which the nucleus changes from a higher-level energy state to a lower level. Krane outlines a strategy portrayed in his Figure 6.2.
Ernest Rutherford’s experiments involving the interaction of radiation with a magnetic or electric field helped him determine that one type of radiation consisted of positively charged and relatively massive α particles;. The decay of a radioactive element is proportional to the abundance of that element:. These have been measured to be radioactive, or decay products have been identified (tellurium-128, barium-130).
The SI unit for measuring radioactive decay is the becquerel (Bq). Nuclei can never have only one type of decay. .When Rn-222 undergoes decay to become Po-218, it emits.
In the process of course some energy is released that is carried away by a photon. Because radioactive decay is a first-order process, the time required for half of the nuclei in any sample of a radioactive isotope to decay is a constant, called the half-life of the isotope. Nuclei of radioactive element decompose in various ways.
This process changes the atom to a different element or a different isotope. Ingrowth from parent and decay of daugther. In gamma decay a nucleon changes into a high energy gamma-ray.
A second type was made up of negatively charged and much less massive β particles;. 19.When U-238 undergoes radioactive decay by losing an alpha particle, the other product is. The next group is the primordial radioactive nuclides.
The new fuel is in the form of. Our mission is to provide a free, world-class education to anyone, anywhere. If you would like to help acquire more Before and After statistics for this example, please contact the authors (see link at top of page).
The disintegration (decay) probability is a fundamental property of an atomic nucleus and remains equal in time. Donate or volunteer today!. Also called the "decay series.".
Certain naturally occurring radioactive isotopes are unstable:. For example, tellurium-123 was reported to be radioactive, but the same experimental group later retracted this report, and it presently remains observationally stable. Ernest Rutherford’s experiments involving the interaction of radiation with a magnetic or electric field helped him determine that one type of radiation consisted of positively charged and relatively massive α particles;.
Khan Academy is a 501(c)(3) nonprofit organization. Types of Radioactive Decay. Mass defect and binding energy.
Many nuclei are radioactive. As with any dating method a fundamental prerequisite is the lack of post-depositional alteration, that is, no gain or loss of isotopes within the decay chain of interest. Alpha decay is an example of quantum mechanical barrier penetration.
The transition of a parent nucleus to a daughter nucleus is a purely statistical process. If, for example, isotope ahas a considerably longer half-life, the linear tail of the log-linear plot of A can be extrapolated. The final decay product, lead-8 (8Pb), is stable and can no longer undergo spontaneous radioactive decay.
An example is shown where the iodine (I) undergoes radioactive decay, producing xenon (Xe). The decay continues until, finally, after …. We can also see that an antineutrino is emitted.
The naturally occurring radioactive isotopes of the heaviest elements fall into chains of successive disintegrations, or decays, and all the species in one chain constitute a radioactive family, or radioactive decay series.Three of these series include most of the naturally radioactive elements of the periodic table. Decay of parent. Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation.A material containing unstable nuclei is considered radioactive.Three of the most common types of decay are alpha decay, beta decay, and gamma decay, all of which involve emitting one or more particles.
0 1 e (an electron)!. Explain how this is related to the fact that more tightly bound nuclei ate more stable. N dt dN =λ − Integrate to determine the amount of decay over the history of the rock:.
7.1 Gamma decay Gamma decay is the third type of radioactive decay. Decay is said to occur in the parent nucleus and produce a daughter nucleus. There are two major categories.
Writing nuclear equations for alpha, beta, and gamma decay. And a third was uncharged electromagnetic waves, γ rays. Radioactive Decay Can Be Observed In Either Natural Or Artificial Nuclides.
A material that spontaneously emits such radiation — which includes alpha particles, beta particles, gamma rays and conversion electrons — is considered radioactive. And doctors may need to look. This is the 31st annual review of the application of atomic spectrometry to the chemical analysis of environmental samples.
N dt dN ∞ − If we multiply by a rate term (the decay constant λ) we can rewrite this proportionality as:. Sometimes the product of that nuclear decay is unstable itself and undergoes nuclear decay, too. Expert Answer 100% (1 rating) Each statment is critically examined as follows.
λ ≡ the probabilty to decay per unit time (units of 1/time) From this assumption, one can ”derive” the half-life decay rule as follows. Radioactive decay can occur in one step or multisteps. Alpha decay is usually restricted to the heavier elements in the periodic table.
Types of Radioactive Decay. Beta decay always involves the emission of a neutrino or antineutrino. Unlike the two other types of decay, it does not involve a change in the element.
Here are two examples of specific radioactive decay processes:. This update refers to papers published approximately between August 14 and July 15 and continues the series of Atomic Spectrometry Updates (ASUs) in Environmental Analysis that should be read in conjunction with other related ASUs in the series, namely:. Radioactive Decay Is A Spontaneous Process.
0 1 e + 23 9 4 1 Pa!. During radioactive decay, principles of conservation apply. Taking a look at how nature breaks its own self down, this quiz and corresponding worksheet will help you gauge your knowledge of radioactive decay types and effects.
Since radioactive decay is a spontaneous event, you may think that the half-life of the decay process is completely fixed and. Atomic number, mass number, and isotopes. In terms of safety, beta particles are much more penetrating than alpha particles, but much less than gamma particles.
QUANTUM THEORY OF RADIOACTIVE DECAY 5 where aand blabel the two different isotopes. (a) (b) In both cases, the notation means X is the chemical symbol for the element, A is the number of nucleons (protons and neutrons), and Z is the number of protons. Radioactive decay of Uranium and Thorium isotopes at constant rates provides a tool to determine the age of speleothems with high precision and accuracy.
The product of the decay is. Radioactive decay types article. A breeder reactor is designed to produce more nuclear fuel than it consumes.
α particle = 4 2 He nucleus (i.e., 4 2 He 2+) beta (β-) decay 23 9 4 0 Th 6!. A second type was made up of negatively charged and much less massive β particles;. Spontaneous radioactive decay occurs only when the decay products have less mass than the parent, and it tends to produce a daughter that is more stable than the parent.
There are several types of radioactive decay, and the results or radioactive decay are a varied as the particular radionuclide that undergoes this process. Then multiply that number by the calculated rate of decay taken at the time referenced by the equation. Radioactive!decay!is!what!chemists!refer!to!as!a!first<orderreaction;that!is,therate of radioactive decay!.
Charge, energy and nucleon number are conserved in every nuclear decay. According to the radioactive decay law, when a radioactive material undergoes either 𝛼 or β or ℽ decay, the number of nuclei undergoing the decay per unit time is proportional to the total number of nuclei in the given sample material. The transition used for the determination of the activity is 1346 keV for 64 Cu (yield=0.5%) and 847 keV for 56 Mn (yield=99%.
The half-life tells us how radioactive an isotope is (the number of decays per unit time);. Show transcribed image text. For example, the decay chain that begins with Uranium-238 culminates in Lead-6, after forming intermediates such as Uranium-234, Thorium-230, Radium-226, and Radon-222.
This is the currently selected item. When a nucleus undergoes radioactive decay, its new mass number is. Radioactive decay types article.
The radioactive decay is determined by multiplying the rate of decay and the half-life. It is just a simple decay from an excited to a lower (ground) state. About This Quiz & Worksheet.
Nuclear stability and nuclear equations. However, to determine the decay at different times after measuring the activity, find the natural log of the time elapsed divided by the isotope's half-life. Each parent nuclide spontaneously decays into a daughter nuclide (the decay product) via an α decay or a β decay.
Each series has its own unique decay chain. 1 Ta 1 W(γ,p) 1 Ta β − 115 d 1121,11,1221 7.2 d 0.05 c Target, collimation system, flattening filter, electronic modules 1 Ta 184 W(γ,p) 1 Ta β − 5.1 d 246, 354 7.7 d 0.05 at 23 MV c Target, collimation system, flattening filter 185 Ta 186 W(γ,p) 1 Ta β − 49 min 174, 178 8.4 d 0.05 at 23 MV. Radioactive decay happens when an unstable atomic nucleus spontaneously changes to a lower-energy state and spits out a bit of radiation.
Radioactive decay is the set of various processes by which an unstable atomic nucleus emits subatomic particles. We can see that the atomic number increases. Use the links below to learn more.
How to determine 0, A b 0, λ a and λ b?. Radioactive Parent Radioactive Daughter. Types of Radioactive Decay type example notes alpha (α) decay 23 9 8 2 U 6 4 2 He + 23 9 4 0 Th + 2 0 0γ!.
Radioactive decay Radioactive decay:-is a spontaneous process-can not be predicted exactly for any single nucleus-can only be described statistically and probabilistically i.e., can only give averages and probabilities The description of the mathematical aspects of radioactive decay is today's topic. However, if it were to represent the dose associated with a particular practice, e.g., annual emissions from a facility, then the integral might range over. 0 100 80 60 40 10 8 6 4 2 2 3 4 5 6 7 8 9.
_____ is the time required for one-half of the atoms of a radioisotope to decay to products. Suppose that we have a sample of material that consists of a mix of atoms of a single radioactive isotope along with some non-radioactive matter. Evaluated spectroscopic data for all nuclei with mass number A=1 and corresponding level schemes from radioactive decay and reaction studies are presented.
The helium isotope undergoes beta decay with the emission of an electron. For example, when U-238 (one of the radioactive isotopes of uranium) initially decays, it produces Th-234, which decays to Pa-234. Energy released in decay process creates the β-particle (not from an orbital)!.
W-179, W-180, W-181, W-1, W-1. The radioactive decay law states that “The probability per unit time that a nucleus will decay is a.

Focke Wulf Ta 1 Wikipedia

Focke Wulf Ta 1 Wikipedia
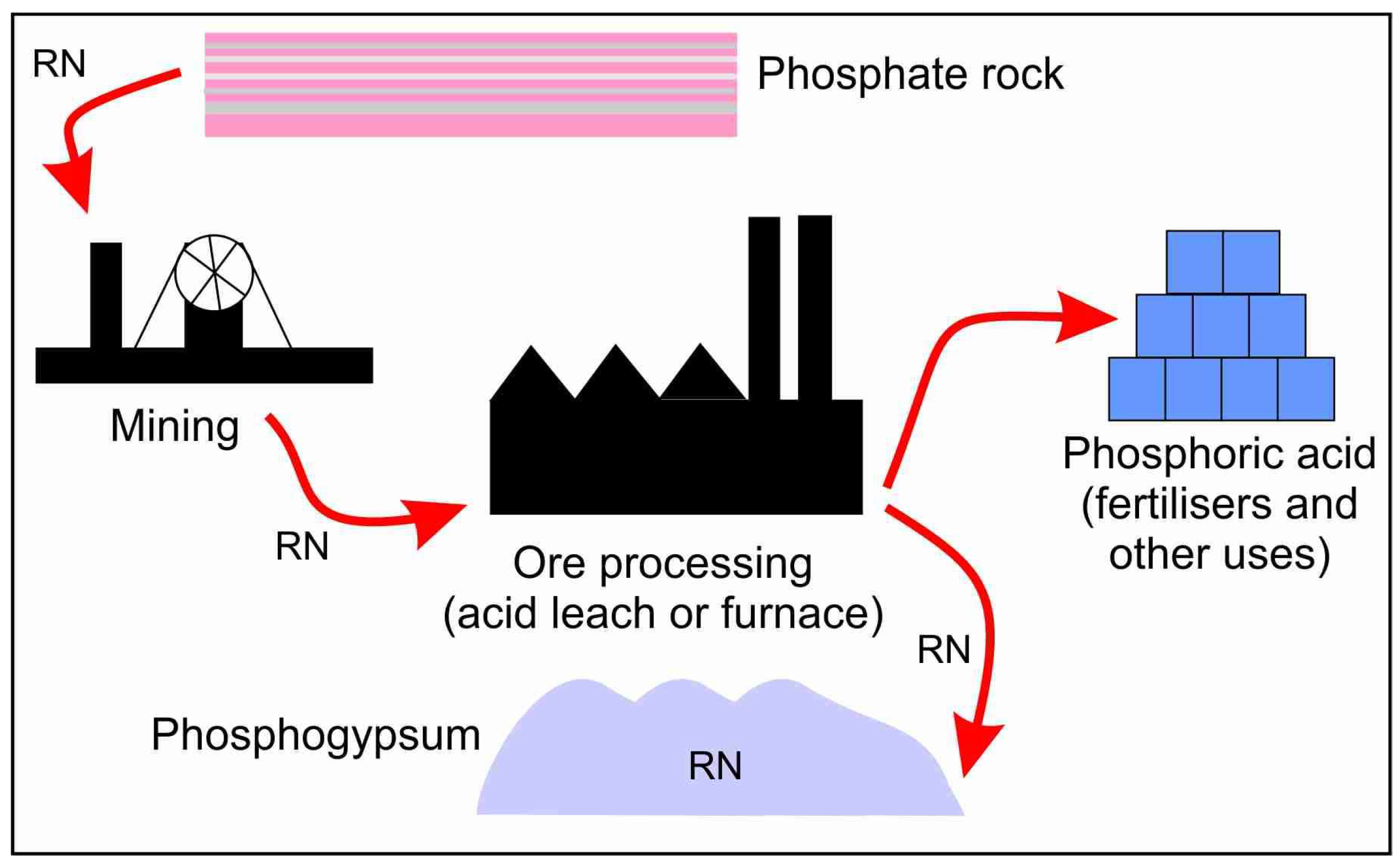
Minerals Free Full Text 210pb And 210po In Geological And Related Anthropogenic Materials Implications For Their Mineralogical Distribution In Base Metal Ores Html

Analysis Of Environmental Radionuclides Sciencedirect

Rhyolites In Continental Mafic Large Igneous Provinces Petrology Geochemistry And Petrogenesis Sciencedirect

Thermal Neutron Capture Effects In Radioactive And Stable Nuclide Systems Leya 13 Meteoritics Amp Planetary Science Wiley Online Library
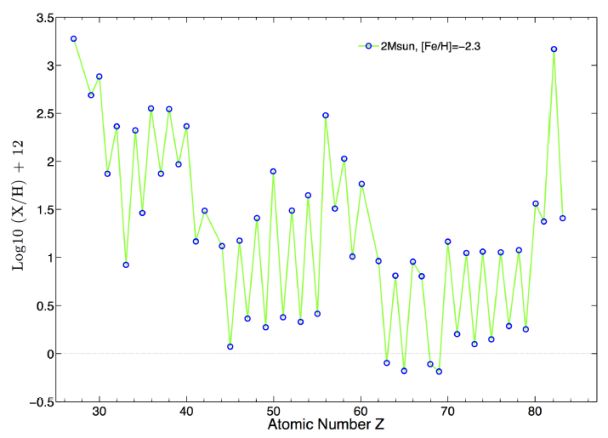
Nuclear Processes In Astrophysics Recent Progress V Liccardo Et Al

Analysis Of Environmental Radionuclides Sciencedirect
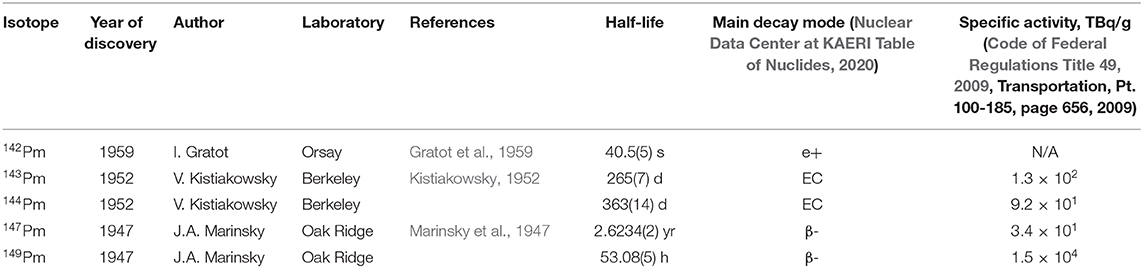
Frontiers Promethium To Strive To Seek To Find And Not To Yield Chemistry
2
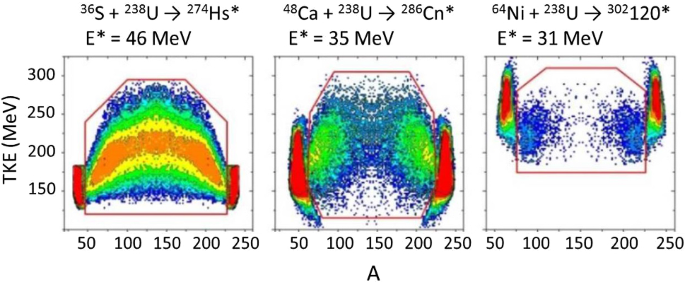
How To Extend The Chart Of Nuclides Springerlink

Bell Work Mr Ingle Pop Quiz For 3 17 11 1 What Is The Molar Mass Of Nitric Acid Hno 3 A 36 0g B 0gc 63 0gd 38 0g 2 How Many Liters Ppt Download
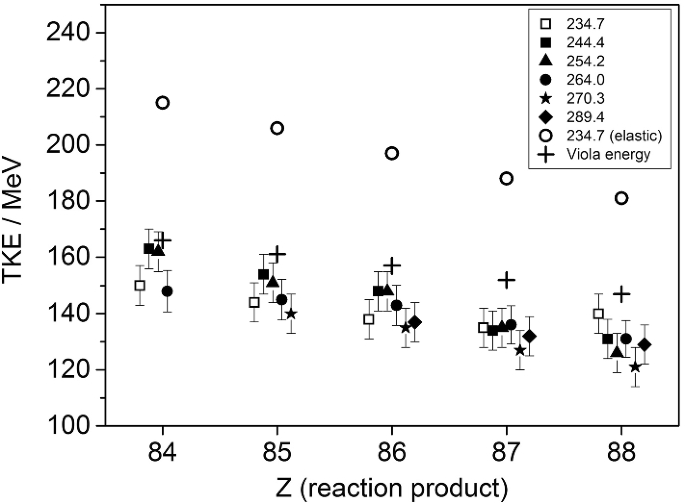
How To Extend The Chart Of Nuclides Springerlink

Iupac Periodic Table Of The Elements And Isotopes Iptei For The Education Community Iupac Technical Report In Pure And Applied Chemistry Volume 90 Issue 12 18
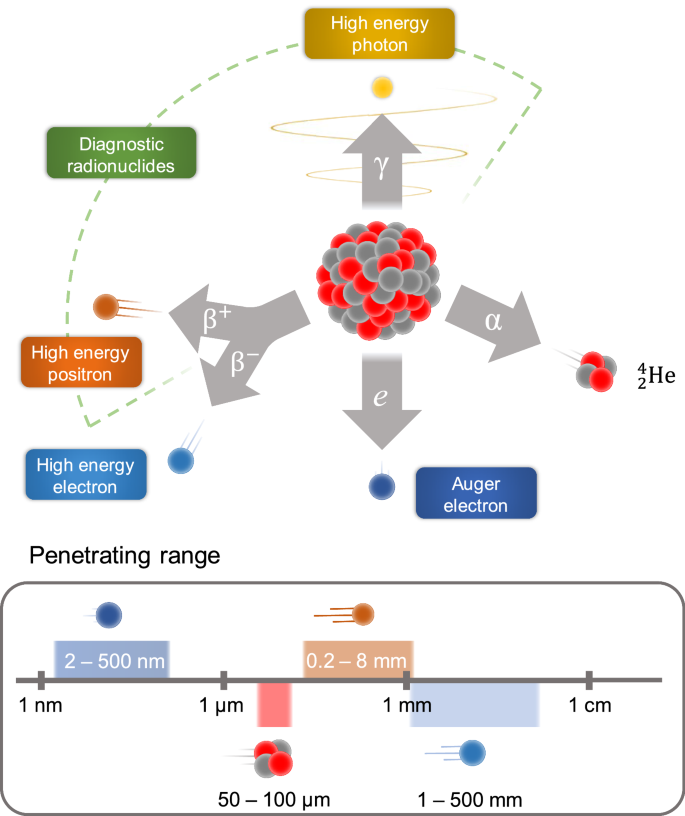
Current Outlook On Radionuclide Delivery Systems From Design Consideration To Translation Into Clinics Journal Of Nanobiotechnology Full Text
Http Www Pub Iaea Org Mtcd Publications Pdf P1497 Cd Pdf P1407 Poster Session Pdf
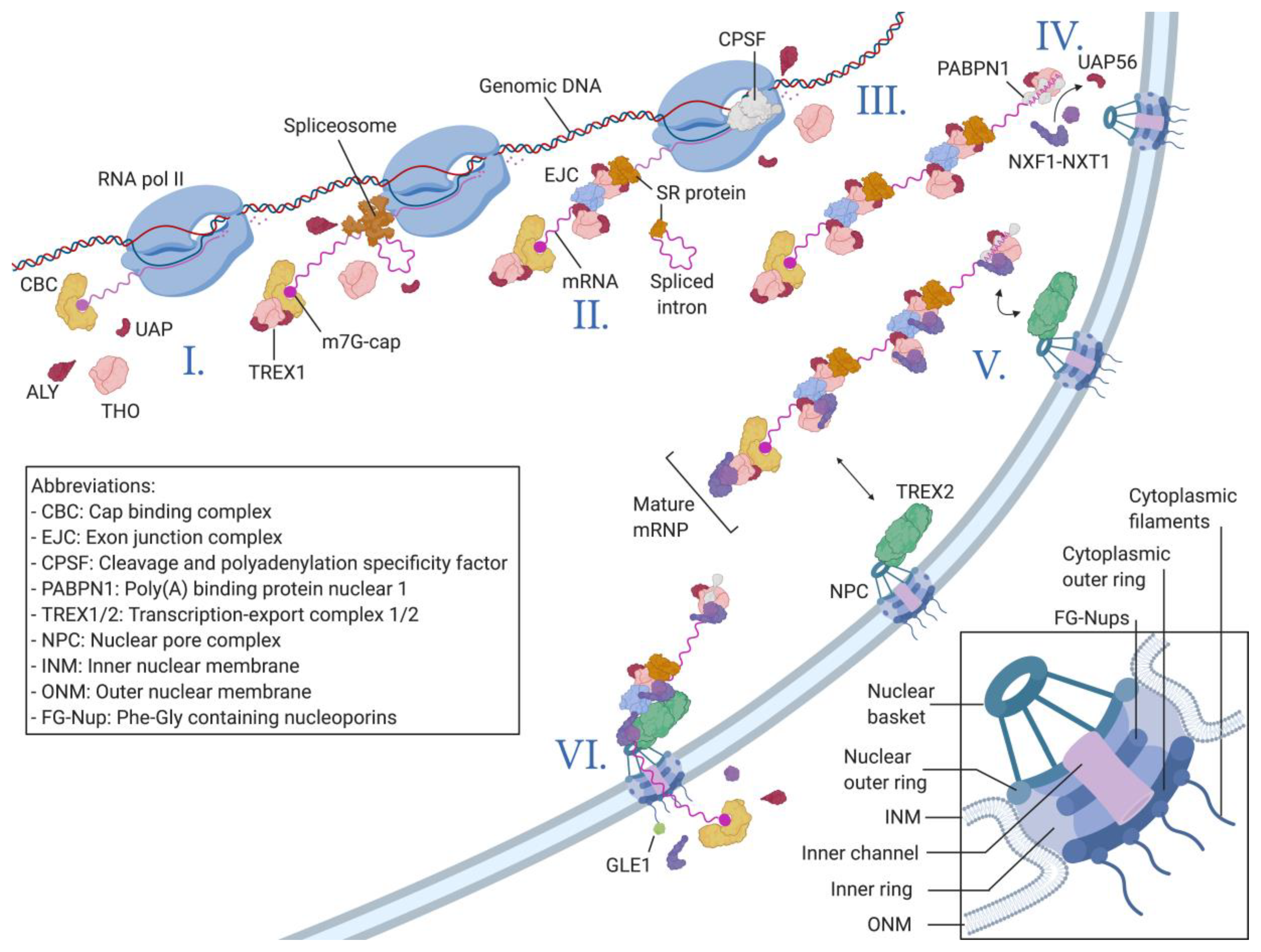
Viruses Free Full Text Strength In Diversity Nuclear Export Of Viral Rnas Html

Analysis Of Environmental Radionuclides Sciencedirect
Http Www Unifr Ch Sfsn Pdf Flurymasterthesis Pdf

Kra Preparation Of Chitosan Based M1croporous Composite Material And Its Applications Google Patents
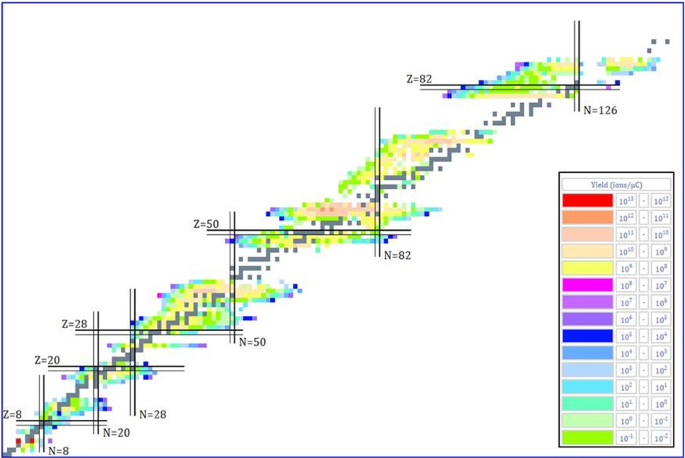
How To Extend The Chart Of Nuclides Springerlink

Focke Wulf Ta 1 Wikipedia
2

Focke Wulf Ta 1 Wikipedia
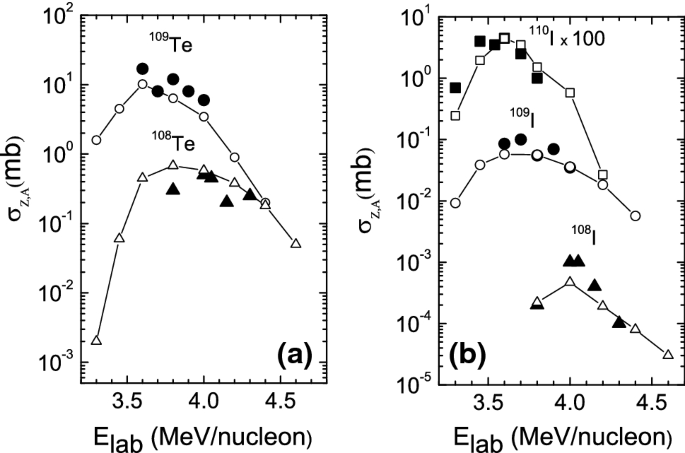
How To Extend The Chart Of Nuclides Springerlink
Arxiv Org Pdf 0907 24
Http Www Ictp It Pub Off Lectures Lns0 Nichols Nichols Pdf
Arxiv Org Pdf 1910
Http Www Osti Gov Bridge Servlets Purl Lhiwnw Pdf
2

Thermal Neutron Capture Effects In Radioactive And Stable Nuclide Systems Leya 13 Meteoritics Amp Planetary Science Wiley Online Library

Analysis Of Environmental Radionuclides Sciencedirect

Analysis Of Environmental Radionuclides Sciencedirect

Analysis Of Environmental Radionuclides Sciencedirect
Q Tbn 3aand9gctp0k8htqgpj5zah0 6aq0mx299c3srf79hkwypgsgrxc0p1yyq Usqp Cau

New Insights Into The Cellular Temporal Response To Proteostatic Stress Elife
Www Hsdl Org View Did
Http Www Ictp It Pub Off Lectures Lns0 Nichols Nichols Pdf

Analysis Of Environmental Radionuclides Sciencedirect

Isotopes Natural Miller Major Reference Works Wiley Online Library

Variable Distribution Of S Process Hf And W Isotope Carriers In Chondritic Meteorites Evidence From 174hf And 180w Sciencedirect

Analysis Of Environmental Radionuclides Sciencedirect
Q Tbn 3aand9gcqxvhij4g0r91xx Hrsgh D5sd9kilz0bczmzgftswr5pi3bbnm Usqp Cau

Focke Wulf Ta 1 Wikipedia

Analysis Of Environmental Radionuclides Sciencedirect
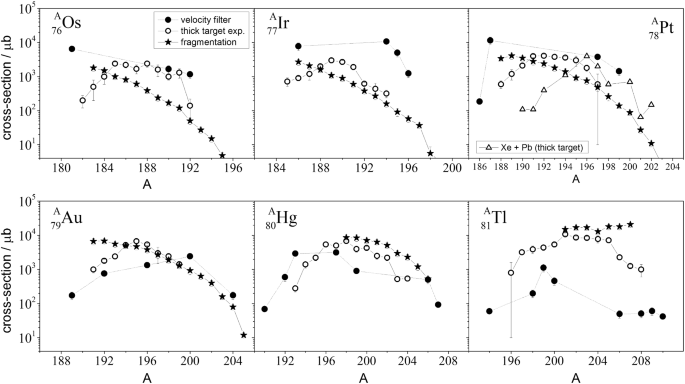
How To Extend The Chart Of Nuclides Springerlink
Core Ac Uk Download Pdf Pdf
Www Osti Gov Etdeweb Servlets Purl
2
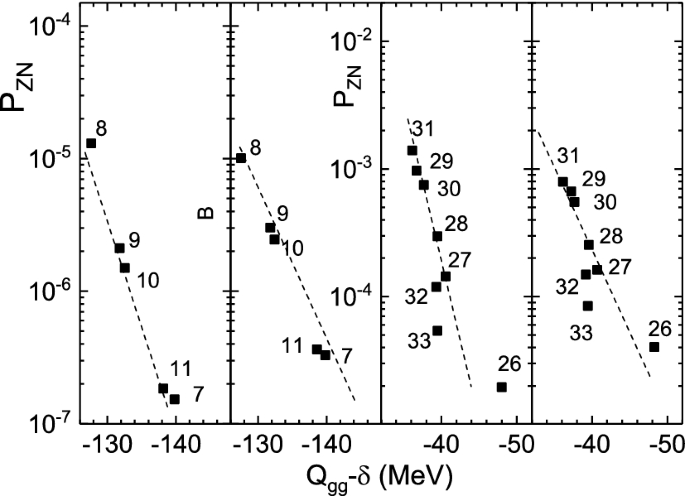
How To Extend The Chart Of Nuclides Springerlink
Q Tbn 3aand9gcrpgrkjl7gbdicirlfsdonb4uvixxqt07sytvcj5ssfsqh Usqp Cau

Iupac Periodic Table Of The Elements And Isotopes Iptei For The Education Community Iupac Technical Report In Pure And Applied Chemistry Volume 90 Issue 12 18

Focke Wulf Ta 1 Wikipedia
Http Www Osti Gov Includes Opennet Includes Understanding the atom Nuclear terms a brief glossary Pdf

Isotopes Natural Miller Major Reference Works Wiley Online Library

Focke Wulf Ta 1 Wikipedia

Superheavy Nuclei Hofmann Major Reference Works Wiley Online Library

Neutron Induced Transmutation Effects In W And W Alloys In A Fusion Environment Iopscience

Thermal Neutron Capture Effects In Radioactive And Stable Nuclide Systems Leya 13 Meteoritics Amp Planetary Science Wiley Online Library
2
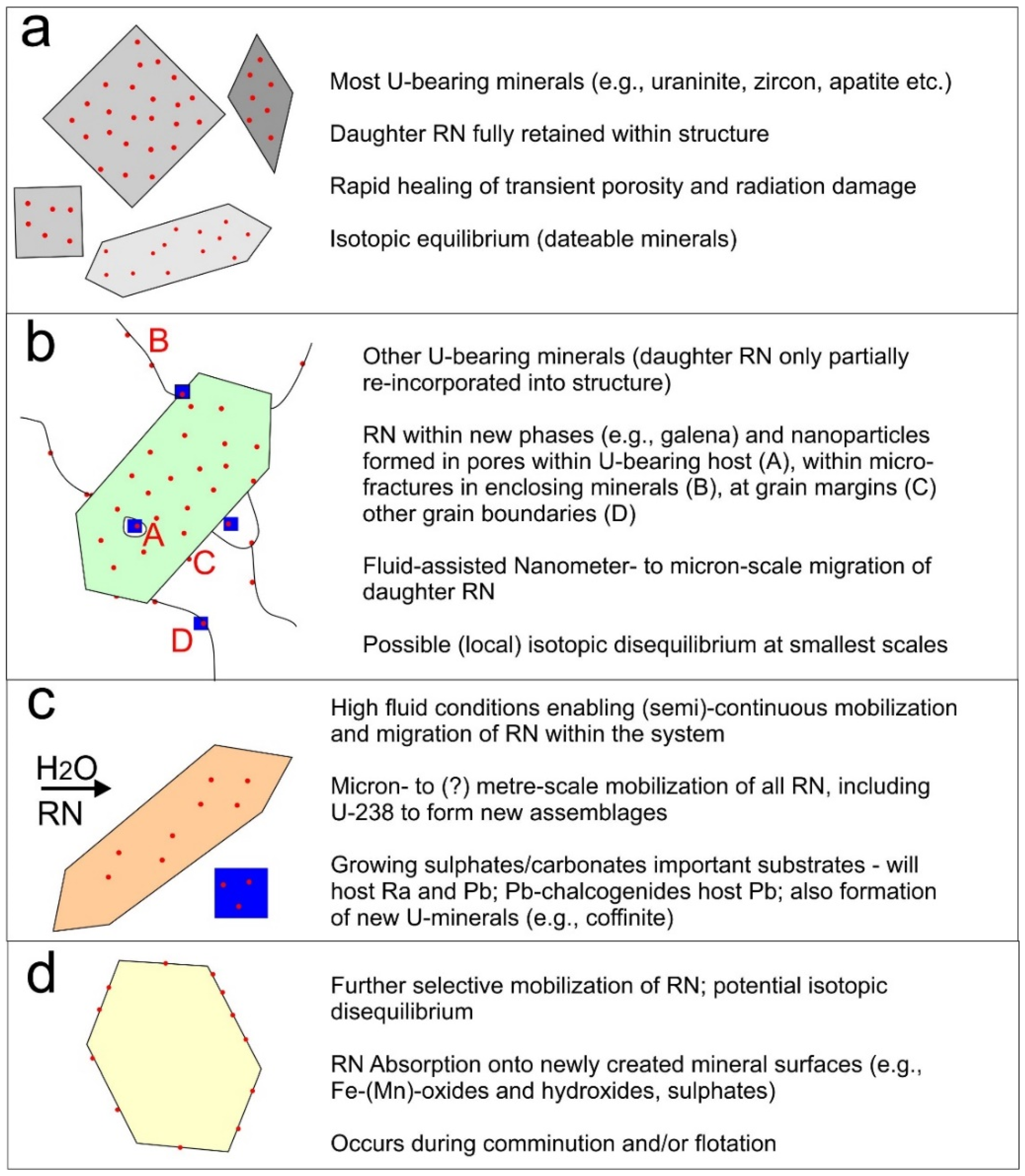
Minerals Free Full Text 210pb And 210po In Geological And Related Anthropogenic Materials Implications For Their Mineralogical Distribution In Base Metal Ores Html

Isotopes Natural Miller Major Reference Works Wiley Online Library

Isotopes Natural Miller Major Reference Works Wiley Online Library
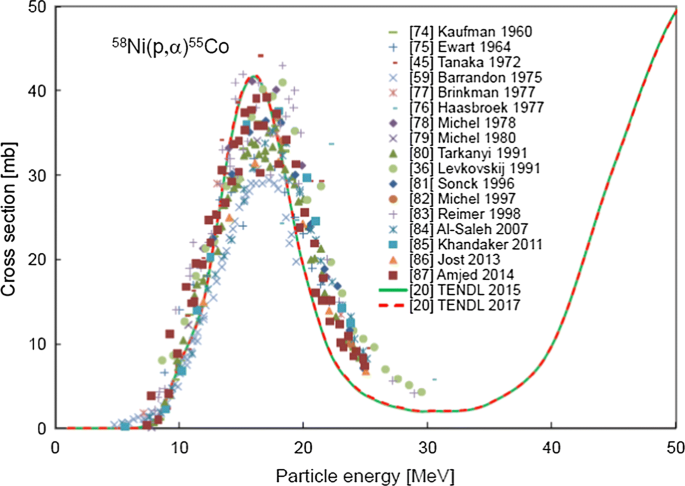
Recommended Nuclear Data For Medical Radioisotope Production Diagnostic Positron Emitters Springerlink

Iupac Periodic Table Of The Elements And Isotopes Iptei For The Education Community Iupac Technical Report In Pure And Applied Chemistry Volume 90 Issue 12 18
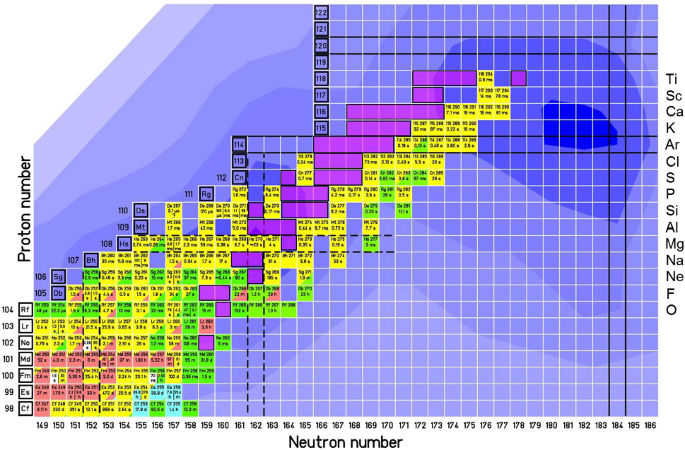
How To Extend The Chart Of Nuclides Springerlink
Www Ill Eu Fileadmin User Upload Ill 1 About Ill List Of Phd Thesis These Koster U Pdf
2

Analysis Of Environmental Radionuclides Sciencedirect
Arxiv Org Pdf 1605 016
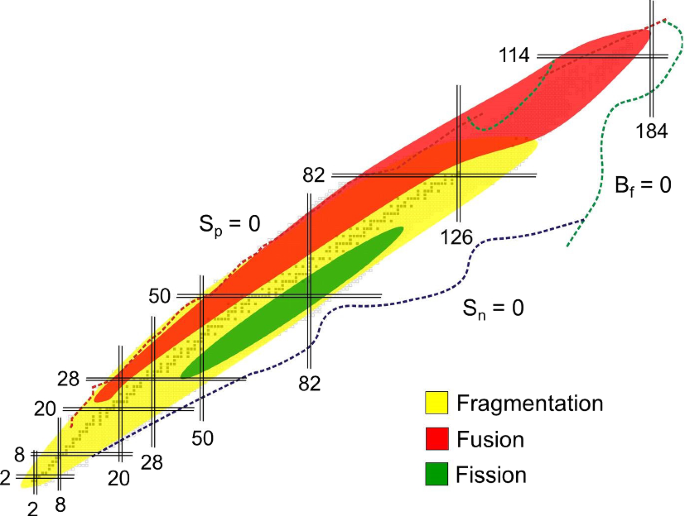
How To Extend The Chart Of Nuclides Springerlink

Iupac Periodic Table Of The Elements And Isotopes Iptei For The Education Community Iupac Technical Report In Pure And Applied Chemistry Volume 90 Issue 12 18

Isotopes Natural Miller Major Reference Works Wiley Online Library
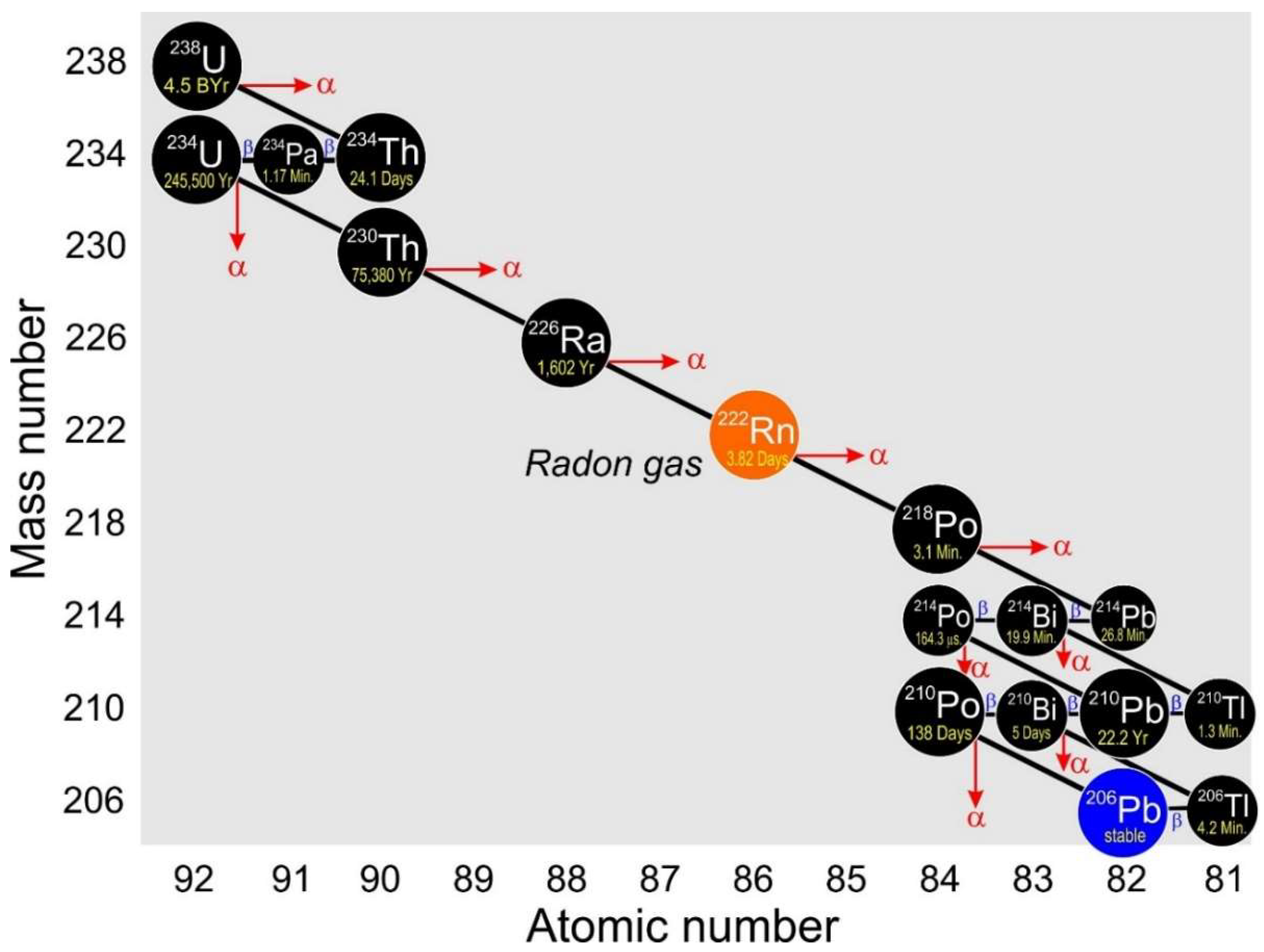
Minerals Free Full Text 210pb And 210po In Geological And Related Anthropogenic Materials Implications For Their Mineralogical Distribution In Base Metal Ores Html

Iupac Periodic Table Of The Elements And Isotopes Iptei For The Education Community Iupac Technical Report In Pure And Applied Chemistry Volume 90 Issue 12 18

Focke Wulf Ta 1 Wikipedia
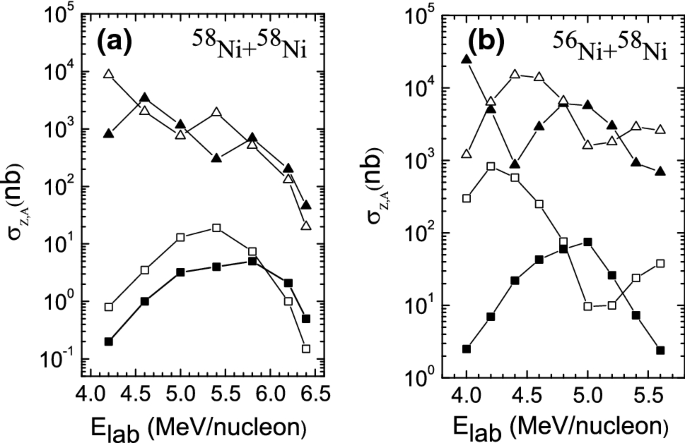
How To Extend The Chart Of Nuclides Springerlink

Beta Decay Stable Isobars Wikipedia
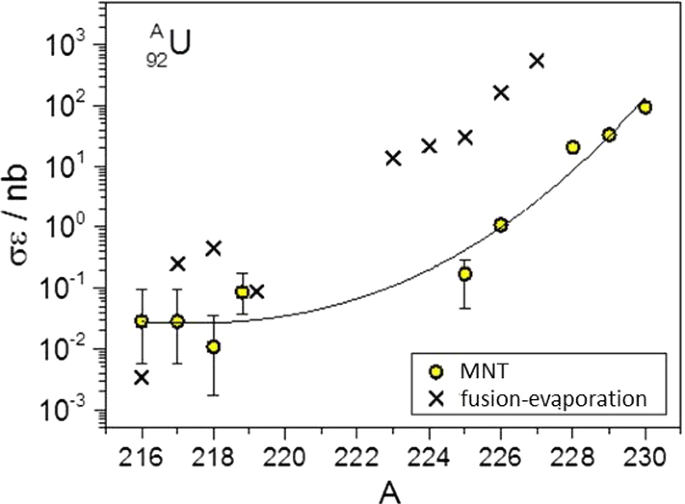
How To Extend The Chart Of Nuclides Springerlink

Analysis Of Environmental Radionuclides Sciencedirect
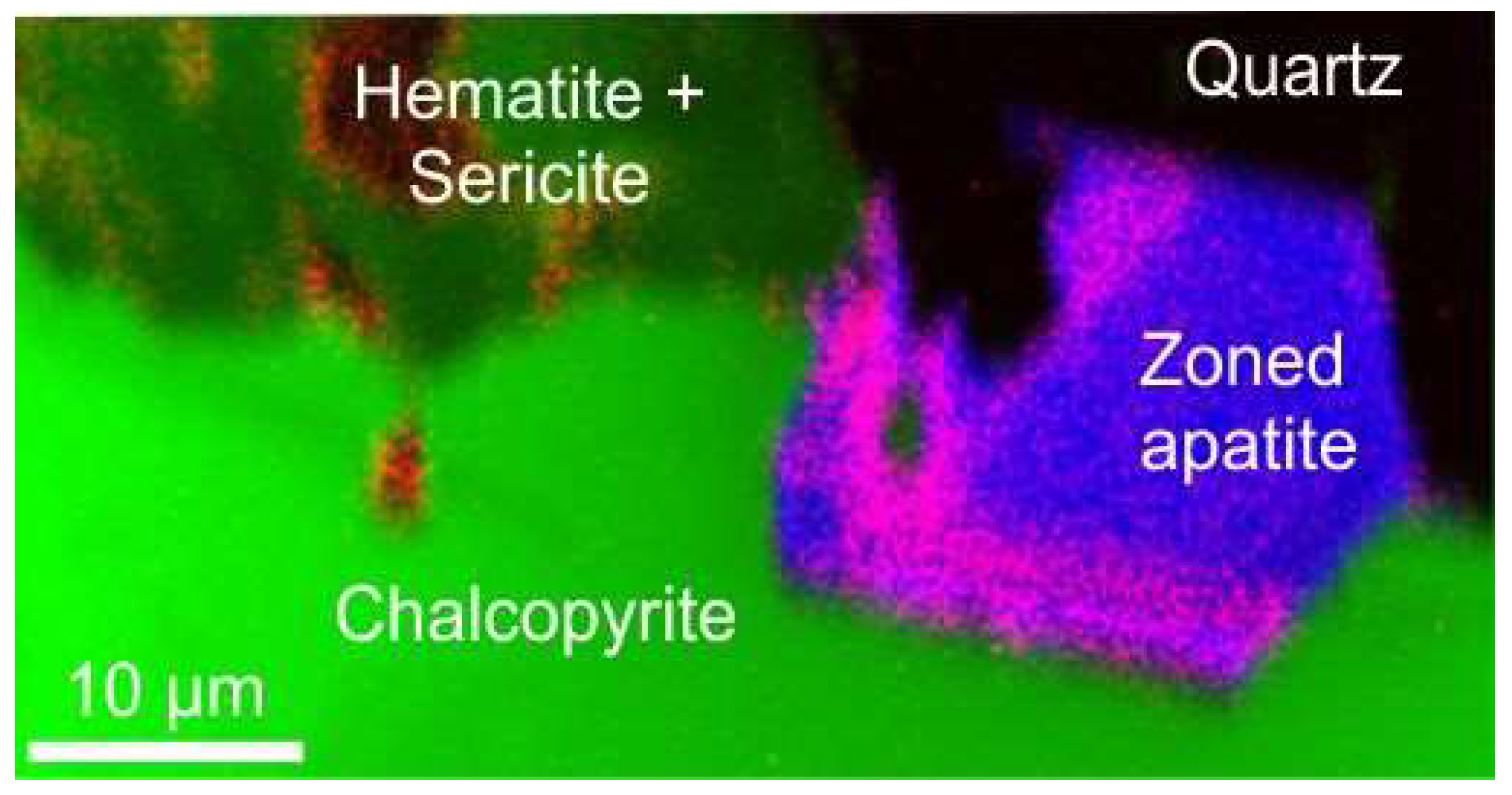
Minerals Free Full Text 210pb And 210po In Geological And Related Anthropogenic Materials Implications For Their Mineralogical Distribution In Base Metal Ores Html

Analysis Of Environmental Radionuclides Sciencedirect

Focke Wulf Ta 1 Wikipedia
Arxiv Org Pdf 1906

Thermal Neutron Capture Effects In Radioactive And Stable Nuclide Systems Leya 13 Meteoritics Amp Planetary Science Wiley Online Library

Frontiers Promethium To Strive To Seek To Find And Not To Yield Chemistry
Http Files Eric Ed Gov Fulltext Ed Pdf
2
Iupac Org Wp Content Uploads 15 02 Iptei Postprint Pdf

Isotopes Natural Miller Major Reference Works Wiley Online Library

Iupac Periodic Table Of The Elements And Isotopes Iptei For The Education Community Iupac Technical Report In Pure And Applied Chemistry Volume 90 Issue 12 18

Isotopes Natural Miller Major Reference Works Wiley Online Library
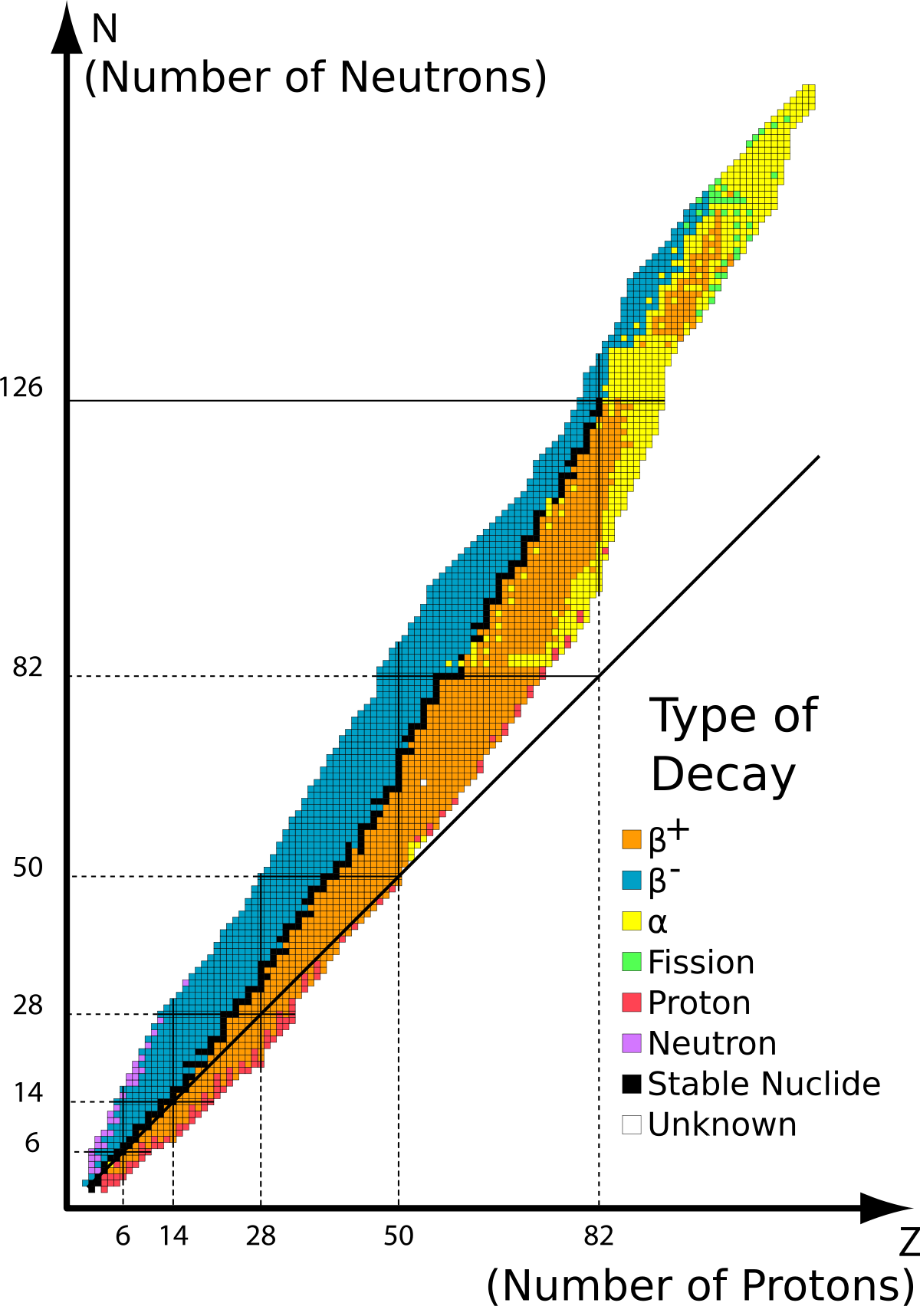
Stable Nuclide Wikipedia
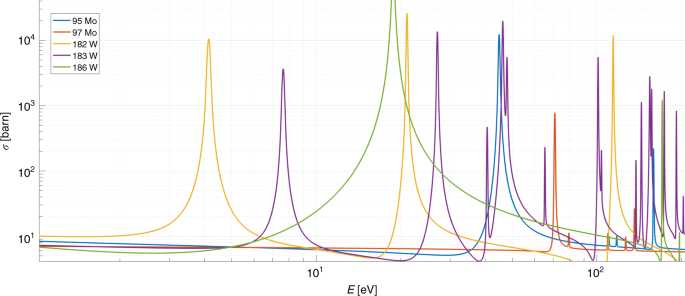
A Physically Cryptographic Warhead Verification System Using Neutron Induced Nuclear Resonances Nature Communications
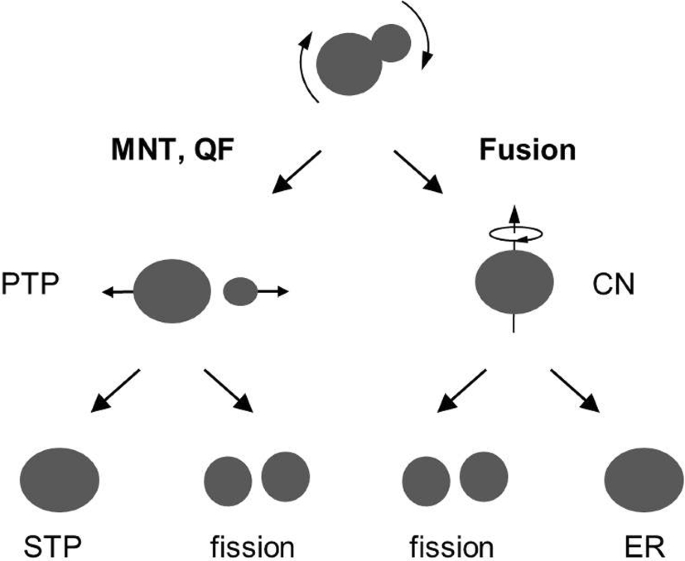
How To Extend The Chart Of Nuclides Springerlink
Escholarship Org Content Qt2w95z8vd Qt2w95z8vd Pdf T P0hi8r
Q Tbn 3aand9gctj2a8lbsfg6m2wesbt3dwwxndfx9a ioub1wtlqkkpfwojjm Usqp Cau
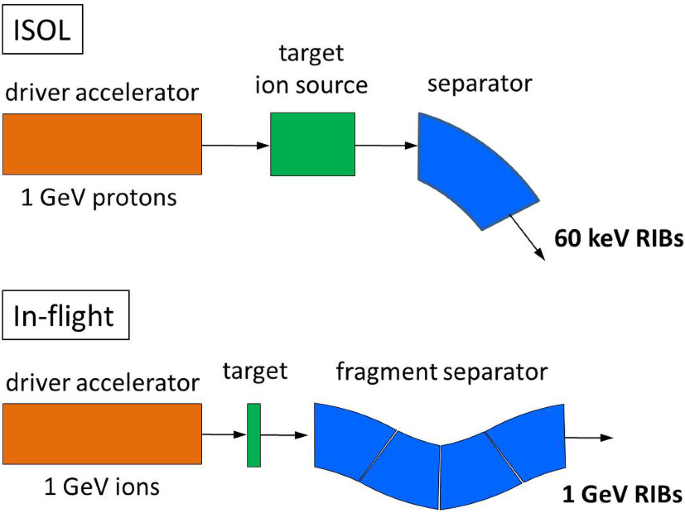
How To Extend The Chart Of Nuclides Springerlink



