One Way To Represent A Model Of An Atom Is In A Drawing What Is Another Way To Show An Atomic Model

How To Draw The Bohr Rutherford Diagram Of Nitrogen Youtube

Bohr Atomic Model
Chemistry Modules

How Are Electrons Distributed In Different Orbits Electronic Configuration

Bohr Atomic Model

Models Of The Atom The Atom Siyavula
The Bohr model and the quantum mechanical model.
One way to represent a model of an atom is in a drawing what is another way to show an atomic model. Nuclear reactions can alter atoms. The Bohr model and the quantum mechanical model. Does the following chemical change represented by these symbols violate any of the ideas of Dalton’s atomic theory?.
These dashed lines represent the different energy levels the electron can have while in the atom. This model depicts an earlier view of the structure of the atom, shortly after the nucleus was discovered. A 3D atom model can be useful to demonstrate in a classroom or use to explain when giving a lesson about atoms.
It’s not unusual to have more than one model represent …. There are a few ways this can be done:. Here's what you need.
While Van den Broek suggested that the atomic number of an element is very similar to its nuclear charge, the latter proposed a Solar-System-like model of the atom, where a nucleus contains the. What is another way to show an atomic model?. In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity.After the cubic model (1902), the plum-pudding model (1904), the Saturnian.
One way to represent a model of an atom is in a drawing. An atom is a building block of matter that cannot be broken apart using any chemical means. The Bohr model and all of its successors describe the properties of.
Limitations of Thomson’s Atomic Model. Niels Bohr’s “Bohr model” is also known as the. 3D atom models are a common science project and craft made to help understand how certain atoms work.
Choose an atom with an atomic number of at least 11, since it has at least three energy level rings source:. Using these models, they investigate the makeup of atoms, including their relative size. Quantum model explains the wave-particle duality of an electron.
Orbital diagrams are a pictorial description of electrons in an atom. A quantum leap in an atom is when a photon carrying just the right amount of energy is absorbed by the atom and its electron jumps to a higher quantum level or higher orbital. If you change the atomic number, you change the element.
The word A’tomos, when translated into English, means:. Bohr was able to predict the difference in energy between each energy level, allowing us to predict the energies of each line in the emission spectrum of hydrogen, and understand why electron energies are quantized. So, if I wrote just the element symbol and its atomic mass on the board that students should be able to figure out the number of particles.
The model proposed by Niels Bohr is the one that you will see in a lot of introductory science texts. Models of the Atom Timeline.This article works well to start because it presents a timeline of atom models right off the bat. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry ().
For example, the most common form (isotope) of carbon-12 is written as:. Each lesson is designed using the 5E method of instruction to …. Main Difference – Thomson vs Rutherford Model of Atom.
Radiation is absorbed or emitted when an electron moves from one orbit to another. The main problem with Bohr's model is that it works very well for atoms with only one electron, like H or He+, but not at all for multi-electron atoms. We like representing these energy levels with an energy level diagram.
Here we see the Thompson (bread pudding) and Rutherford (nuclear) models that lead to the Bohr model, which was extended by Sommerfeld, and finally replaced by Schrödinger’s Quantum Mechanical Model. Think about what that means. Of these two models, the Bohr model is simpler and relatively easy to understand.
Make Comparisons If an atom had a nucleus 1 ft in diameter, what would be the diameter of. It was unable to explain the scattering experiment of Rutherford. Even way back in the time of ancient Greece, they wondered about atoms.
There are two models of atomic structure in use today:. Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. Initially, people did not understand the atom and many scientific investigations were carried out to understand the model of an atom.
Thompson, put forth his ‘Plum Pudding’ Model of an atom. Students also practice adding and subtracting electrons from an atom and determining the overall. Look up the atom on a periodic table and determine the number of electrons present.
Because Ψ 2 gives the probability of finding an electron in a given volume of space (such as a cubic picometer), a plot of Ψ 2 versus distance from the nucleus ( r ) is a plot of the probability density. When you think of an atom, you might picture a little solar system-like model with paths of electrons orbiting a centralized nucleus. How you place them depends on which model of atomic structure your class is studying.
Thomson’s atomic model failed to explain how the positive charge holds on the electrons inside the atom. The splitting of the atom in the th century could most likely not have been accomplished without Dalton laying the foundation of knowledge about the atomic makeup of simple and complex molecules. Up until very recently no one could say one way or another.
So what’s an atom look like?. The relationship between atomic mass units and grams, is that one atomic mass unit has a mass of 1.6 times 10 to the minus 24 grams. Something that cannot be divided any further.
Leave the number set a zero. The model described the atom as a tiny, dense, positively charged core called a nucleus, around which the light, negative constituents, called electrons, circulate at some distance. Two models of atomic structure are in use today:.
The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Quantum model can be used for any atom, including smaller ones and large, complex atoms. Thomson’s Plum Pudding Model was one such description of an atom, which was at that time deemed the most fundamental constituent of matter.
The Structure of the Atom Early Ideas about Matter 1. Thomson in the late 19th century. A Bohr Model is another way to represent an atom, except unlike the Lewis Dot Structure, the Bohr Model shows all of the subatomic particles in an atom.
This model is typically taught to younger students as an introduction to atomic structure. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Draw an image of Thomson’s model of the atom and Rutherford’s model of the atom.
The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This was accurately presented after several scientists came up with different models.
The classic model of an atom was given by Ernest Rutherford called the Rutherford atomic model or Rutherford model of the atom. It also failed to explain an atom’s stability. Could jump from one orbit to another as they gained or lost energy.
The best way to represent the electrons is to draw them as two pairs, and two more single electrons. Also, the students will be able to identify that protons determine an element’s identity. A molecular model, in this article, is a physical model that represents molecules and their processes.
Draw the orbital filling diagram for the atom. In the following drawing, the green spheres represent atoms of a certain element. Rutherford model, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford.
Protons and neutrons form the atomic nucleus. You can find the table in an encyclopedia, a science textbook or online. So there are six neutrons.
The Rutherford model is one of the most popular models of the atom even though it was only considered accurate from 1909 to 1913. The nucleus generally is drawn containing. Quantum model is an atomic model which is considered as the modern atomic model to explain the structure of an atom accurately.
The atomic number doesn't change when you're talking about an isotope. The purple spheres represent atoms of another element. The creation of mathematical models of molecular properties and behaviour is molecular modelling, and their graphical depiction is molecular graphics, but these topics are closely linked and each uses techniques from the others.In this article, "molecular model" will primarily refer to.
This model is great for showing how the electrons are placed outside of the nucleus but is not the most recent model of the atom. Thomson had discovered that atoms are composite objects, made of pieces with positive and negative charge, and that the negatively charged electrons within the atom were very small compared to the entire atom. Bohr Model of the Atom In the Bohr model, electrons move around the nucleus like planets around the sun.
Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms. Greek philosophers could not conduct experiments to verify their hypothesis, whereas Dalton could make careful measurements. The proton is a positively charged particle in the nucleus.
Notable Terms Nucleus :. Select an atom from the list (you will probably want to do lower atomic numbers). As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has.
The first person who discovered electrons, Sir J.J. OK let's make that model. We know a structure of an atom consists of electrons, protons, and neutrons.
Make a stack of coins the same height as each number, and line the stacks up next to each other in order (this way you are making a "graph" using stacks of coins, instead of drawing one on paper). What evidence led scientists to accept Rutherford’s model?. Alright let's talk about orbital diagrams.
Bohr Model of Hydrogen The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. If so, which one?. There are a lot of good ideas in this model, but it is not the one that agrees with all of the.
So just subtract the atomic number from the mass number and you'll get the number of neutrons in your atom. A model is useful because it helps you understand what’s observed in nature. He believed that the atom was a uniform sphere with a positive charge and had electrons embedded in it.
The next six electrons go into the second energy level. This became the basis as Dalton’s Law (aka. One way of representing electron probability distributions was illustrated previously for the 1s orbital of hydrogen.
Define atom using your own wor. Before you begin, look through the Periodic Table of Elements and pick an atom. At the end of this atoms lesson plan, students will be able to describe the structure of atoms, including the masses, electrical charges, and locations of protons, neutrons, and electrons.
That means that 100 a hydrogen at, atom, for example, which weighs approximately one atomic mass unit, has a mass of approximately 10 to the minus 24 grams. A quantum is the amount of energy needed for an electron to move from one energy level to another. The theory did not mention anything about the nucleus of an atom.
Contrast the methods used by the Greek philosophers and Dalton to study the atom. The Bohr model explains the particle behavior of an electron. Click on the “Calculate” button and compare your answer with the one provided.
The Discovery of the Electron When a voltage is applied between a positive and negative electrode placed in a completely evacuated or vacuumed chamber, the glass or the region of the chamber behind. The nucleus is the center of an atom with. If the spheres touch, they are part of a single unit of a compound.
Let's do another one. In order to figure out where electrons go in an atom we have to follow 3 main rules. A model is useful because it helps you ….
The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. An early model of the atom was developed in 1913 by Danish scientist Niels Bohr (15–1962). This is the early atomic model that Niels Bohr and.
Bohr model can be applied for hydrogen atom but not for large atoms. The light specra of atoms is evidence of Bohr's atomic model-because light gets emitted from an atom in quantized (fixed) amounts. The quantum mechanical model is based on mathematics.
Chadwick discovered the. Thomson model of atom is one of the earliest models to describe the structure of atoms.This model is also known as the plum pudding model due to its resemblance to a plum pudding. That’s where the word comes from, ancient Greece.
The first two electrons go into the first energy level. Since we know the atomic number is 6 (because we memorized it), the atom has 6 protons. Dalton’s law of partial pressures), which stated that in.
The ‘Plum Pudding Model’ is one of the many theories that were hypothesized to explain atomic structure, in the beginning of the th century. This explains that this atom is a spherical structure made out of a positively charged solid material and the electrons are embedded in that solid. Students use gumdrops and toothpicks to make lithium atom models.
The question of what makes up an atom, was explored by many scientists at the turn of the 19 th century. Students are then asked to form molecules out of atoms, much in the same way they constructed atoms out of the particles that atoms are made of. Each element, when electrically neutral, has a number of electrons equal to its atomic number.
Once again, the atomic theory was revised to account for new data. This is carbon and this time we have a superscript of 13. The Plum Pudding Model is a model of atomic structure proposed by J.J.
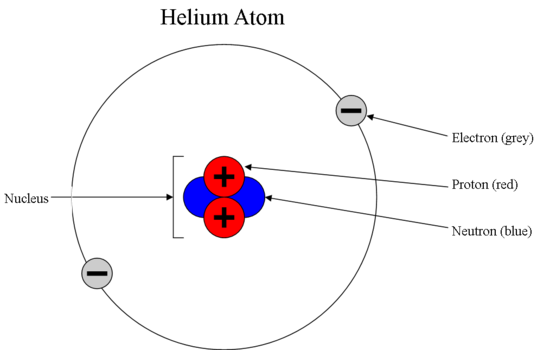
It can be delivered by a photon. A depiction of the atomic structure of the helium atom.

5 Atom Models Atom Models Bohr Chemistry En Nuclear Plum Pudding Science Solid Sphere Wave Mechanical Glogster Edu Interactive Multimedia Posters
Q Tbn 3aand9gcqohogd3gbirrz0uwnaw8nwat5ds5sdr8h7z6kejcvreb45deon Usqp Cau
Sub Atomic Particles Chemistry Libretexts
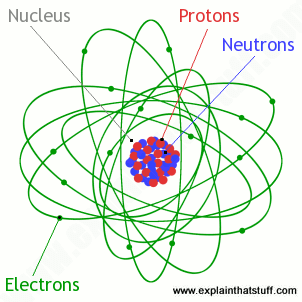
Atoms What Are They What S Inside Them Explain That Stuff

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit

Basic Chemistry Tutorial 2 Drawing Atoms Sciencemusicvideos
Ncert Exemplar Class 9 Science Solutions Chapter 4 Structure Of Atom Access Free Pdf
Questions And Answers How Do I Make A Model Of An Atom

What Is An Atom Live Science
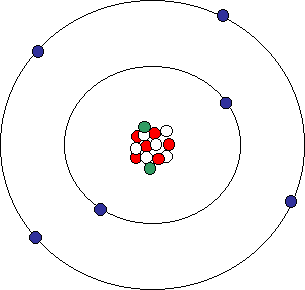
Atomic Structure

Atom Read Chemistry Ck 12 Foundation
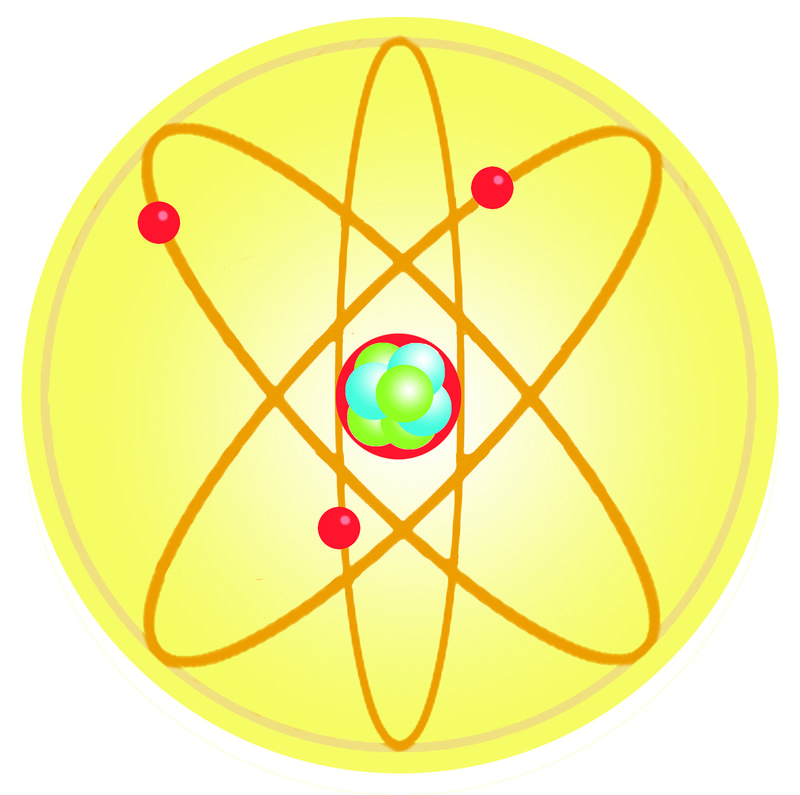
Dalton S Atomic Theory Read Chemistry Ck 12 Foundation

Atomic Models Thomson S Atomic Model And Rutherford S Atomic Model
Chemistry Modules

Atomic Structure And Subatomic Particles Youtube

Structure Of An Atom Learn Chemistry Class 9 Amrita Vidyalayam Elearning Network
How To Draw The Atomic Structure Of Atoms
2 1 Atoms Isotopes Ions And Molecules The Building Blocks Biology 2e Openstax

Atomic Theory Flashcards Quizlet

The Development Of The Atomic Model Wired

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit

The History Of The Atom Theories And Models Compound Interest

Sub Atomic Particles Atoms Siyavula

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit

The Development Of The Atomic Model Wired
Www Sas Upenn Edu Patann 07 04 09 Pogilpdf Contint Pdf

The Structure Of The Atom Astronomy

Sub Atomic Particles Atoms Siyavula
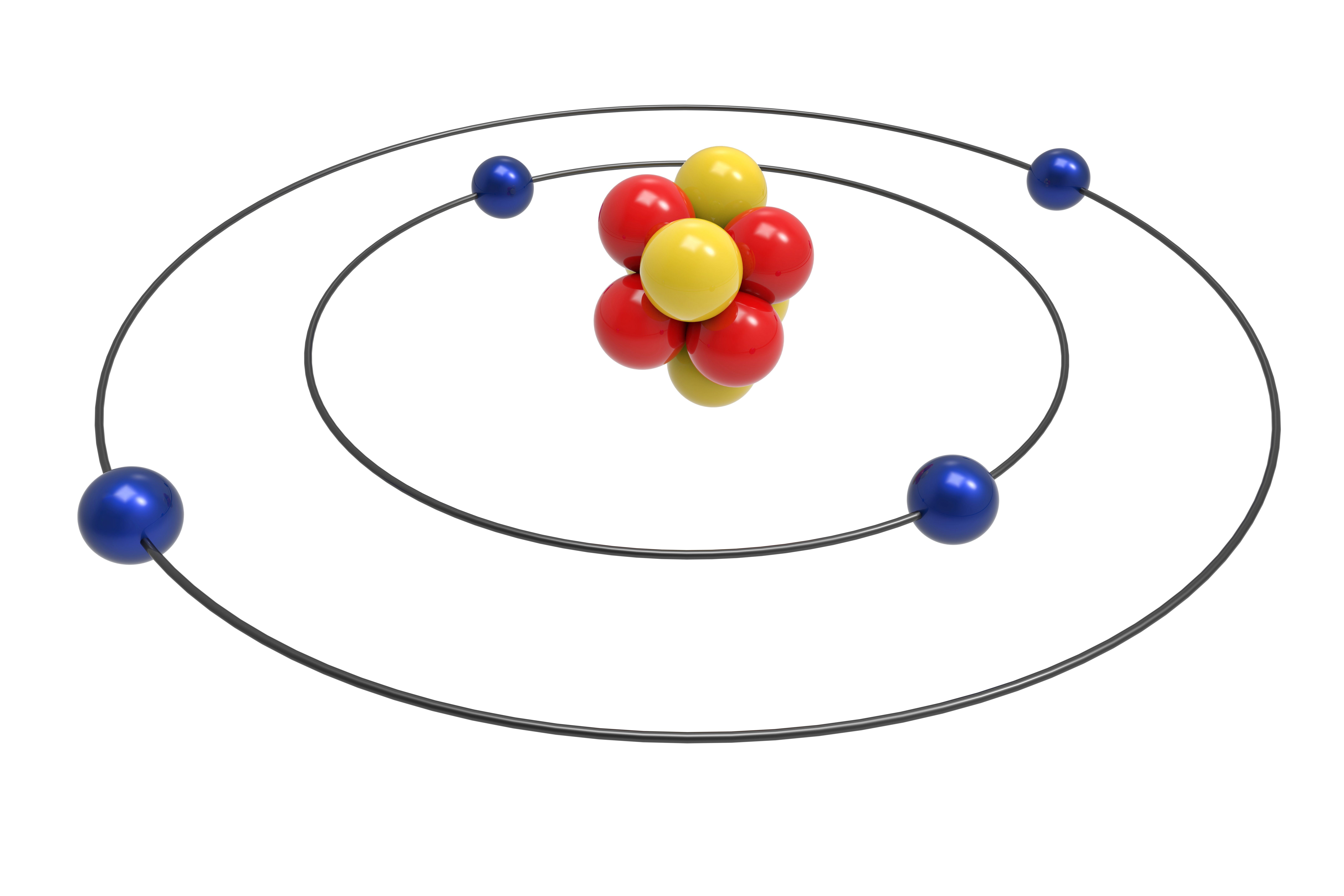
Five Types Of Atomic Models
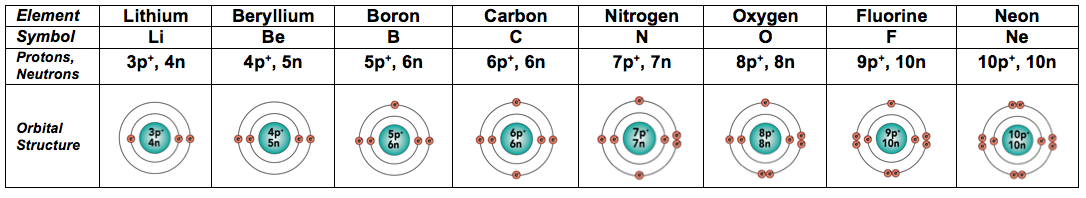
Basic Chemistry Tutorial 2 Drawing Atoms Sciencemusicvideos
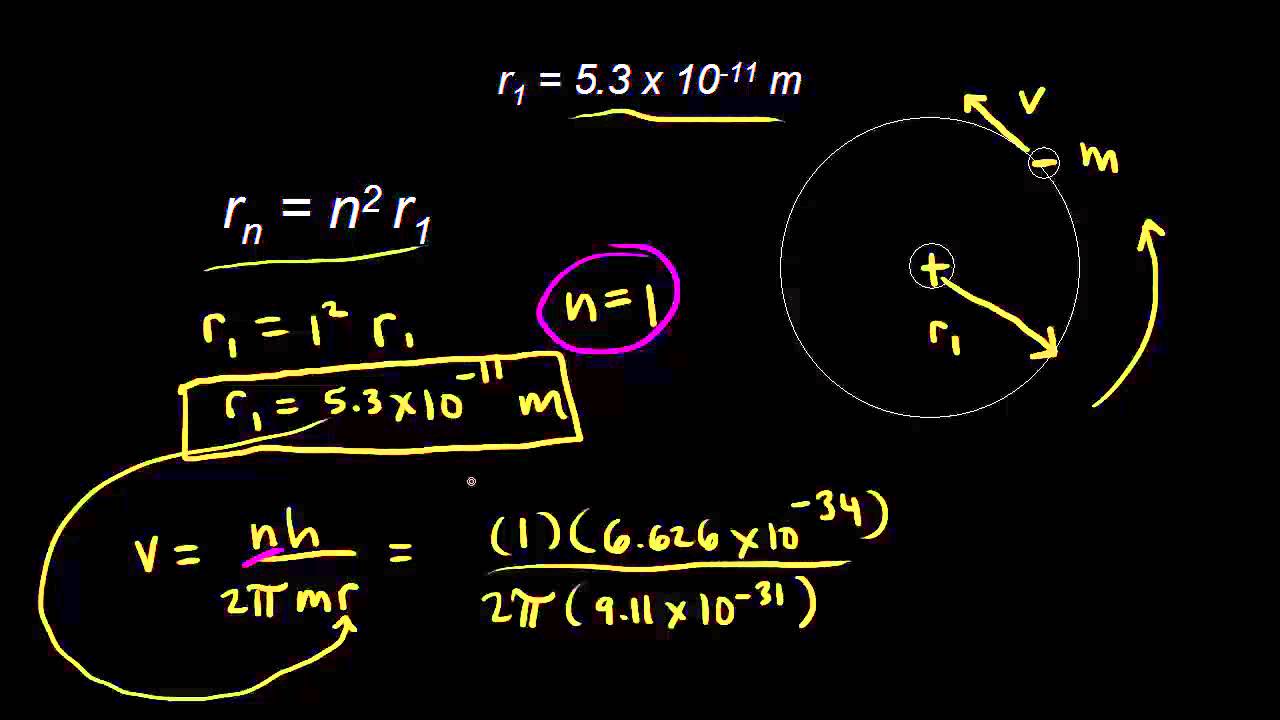
Bohr Model Radii Video Khan Academy
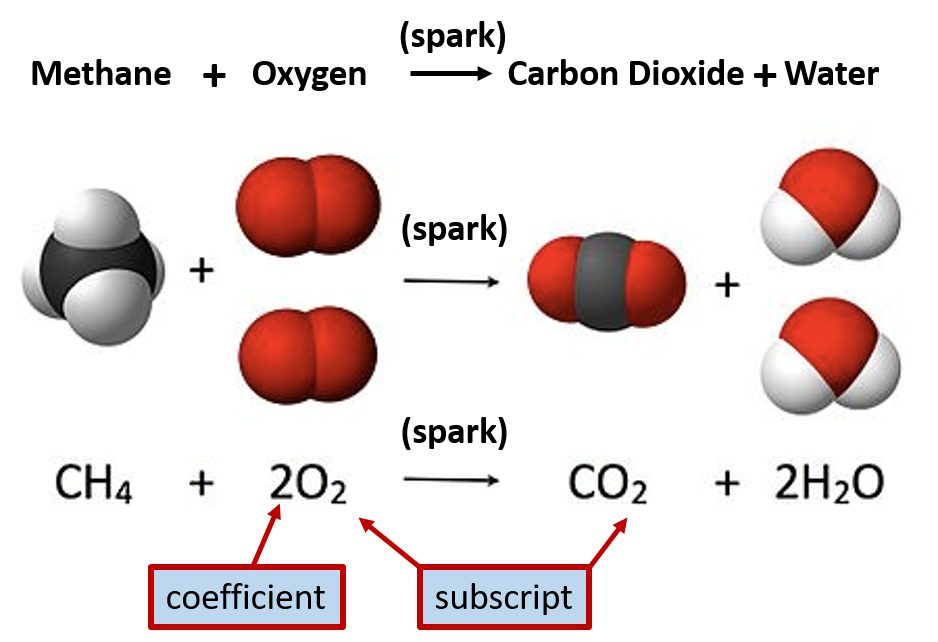
Ch103 Chapter 2 Atoms And The Periodic Table Chemistry
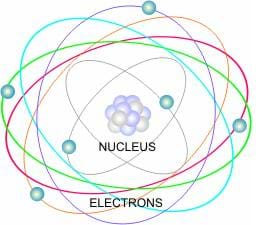
The Building Blocks Of Matter Lesson Teachengineering
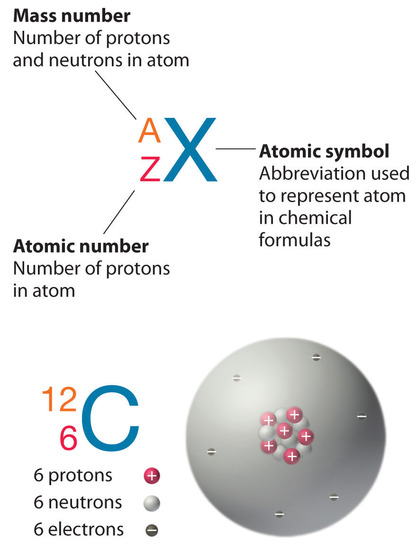
2 3 The Modern View Of Atomic Structure Chemistry Libretexts
3

Atoms Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science
Elements And Atoms Video Khan Academy

Chemistry I Atoms And Molecules

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit

5 Atom Models Atom Models Bohr Chemistry En Nuclear Plum Pudding Science Solid Sphere Wave Mechanical Glogster Edu Interactive Multimedia Posters
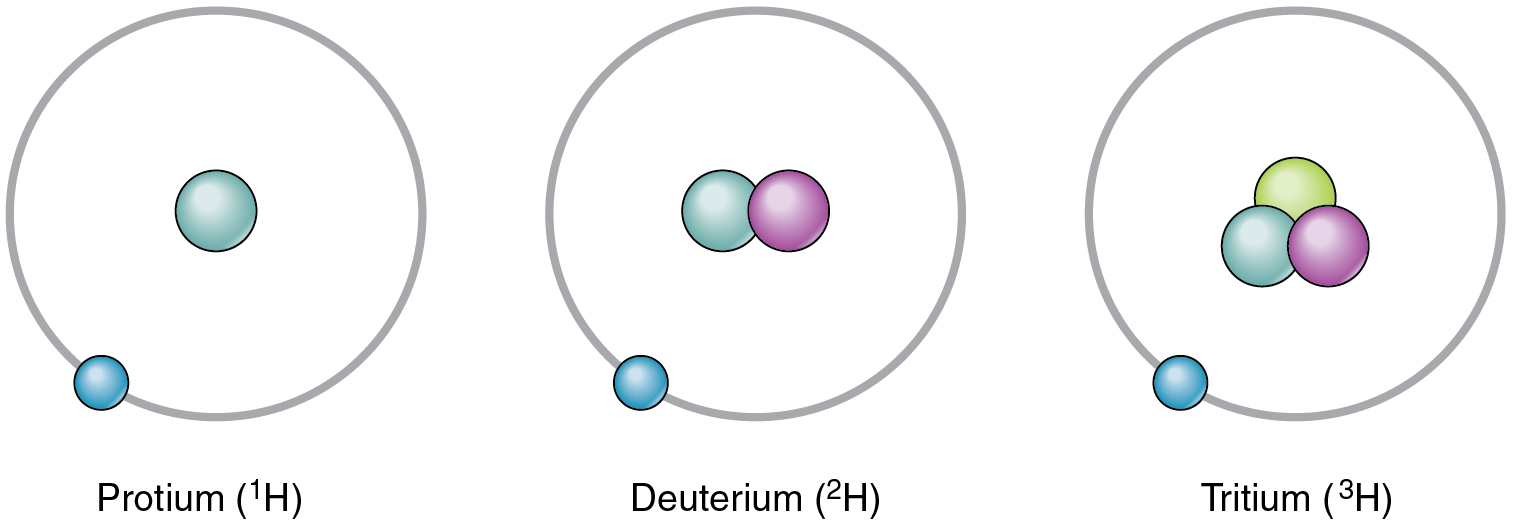
2 1 Elements And Atoms The Building Blocks Of Matter Anatomy And Physiology
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How To Draw A Lewis Structure
Www Sas Upenn Edu Patann 07 04 09 Pogilpdf Contint Pdf
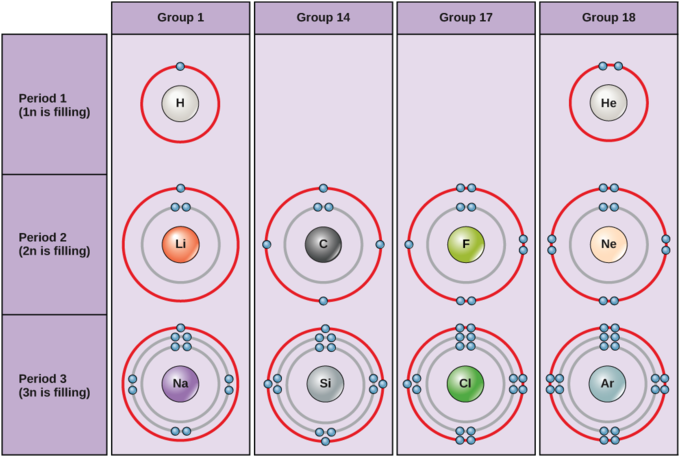
Bohr Diagrams Of Atoms And Ions Chemistry Libretexts

Atomic Models Thomson S Atomic Model And Rutherford S Atomic Model
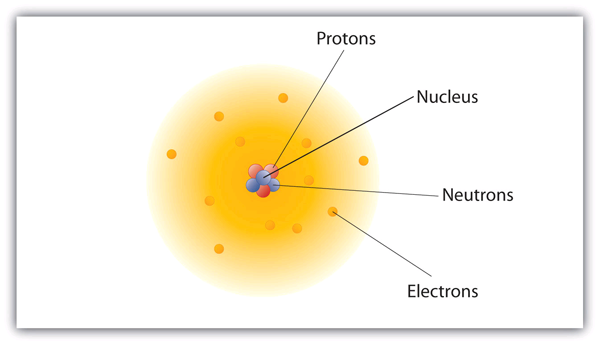
Atomic Theory Introductory Chemistry 1st Canadian Edition

Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy Levels Lyman Balmer Series Youtube
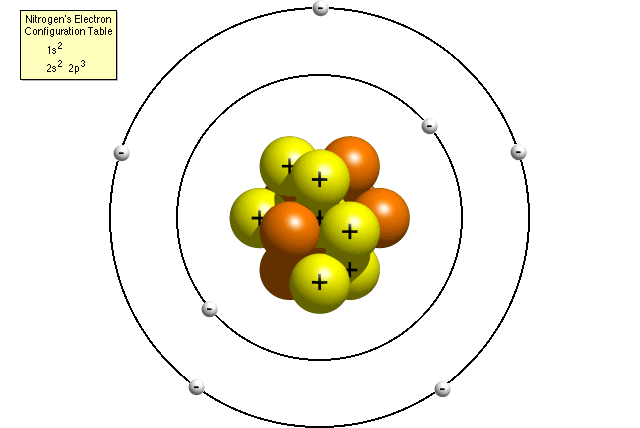
Questions And Answers How Do I Make A Model Of An Atom

Bohr Model Bohr Atomic Model Chemistry Tutorcircle Com Bohr Model Chemistry Projects Science Projects

Atom Wikipedia
Www Sas Upenn Edu Patann 07 04 09 Pogilpdf Contint Pdf
Www Conejousd Org Portals 49 Departments Science Cord 15 Ch 2 matter Honors H hw worksheets no sg Pdf Ver 19 06 06 710

5 Atom Models Atom Models Bohr Chemistry En Nuclear Plum Pudding Science Solid Sphere Wave Mechanical Glogster Edu Interactive Multimedia Posters

How To Draw The Bohr Rutherford Diagram For Calcium Youtube

Quantum Model Of The Atom
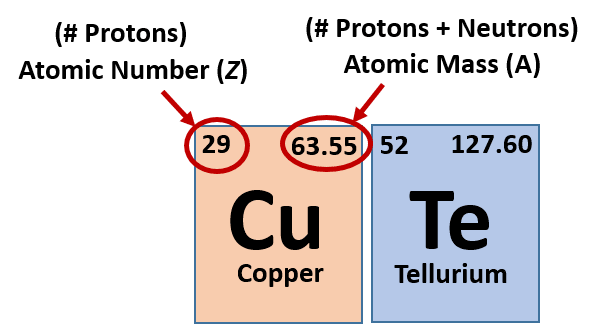
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
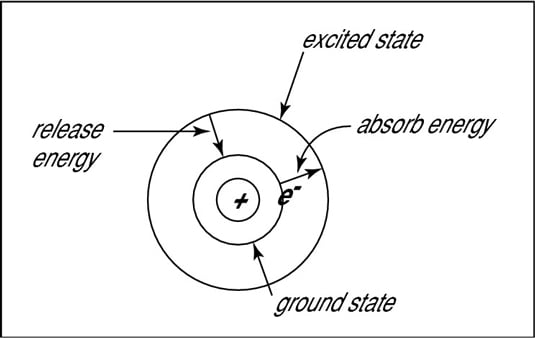
Atomic Structure The Bohr Model Dummies

Chemistry I Atoms And Molecules
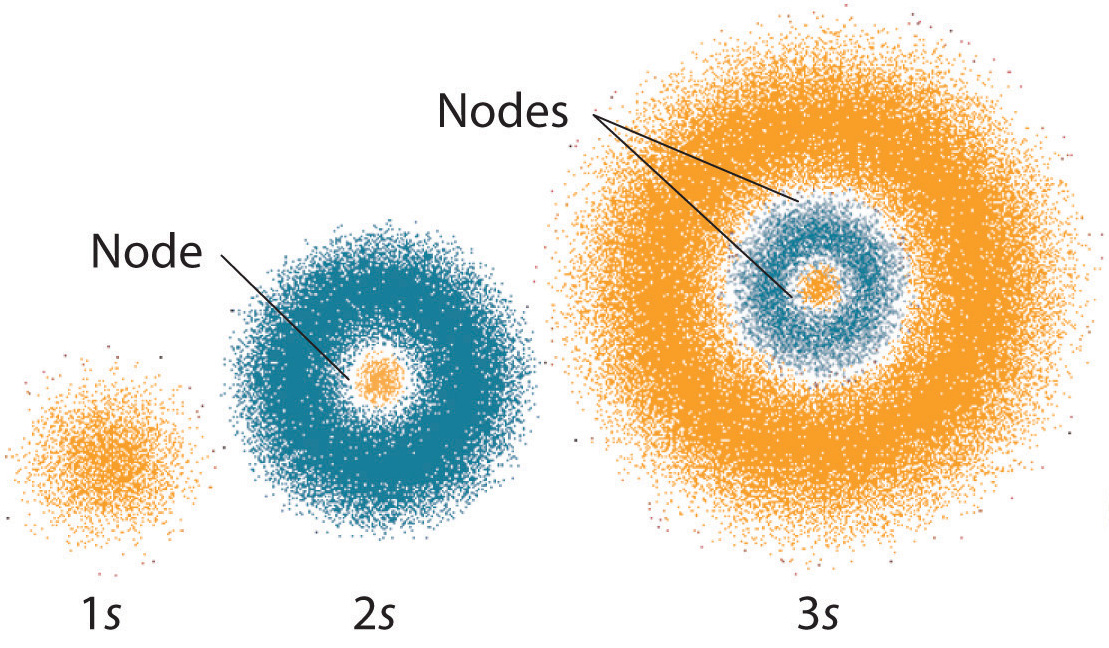
The Quantum Mechanical Model Of The Atom Article Khan Academy
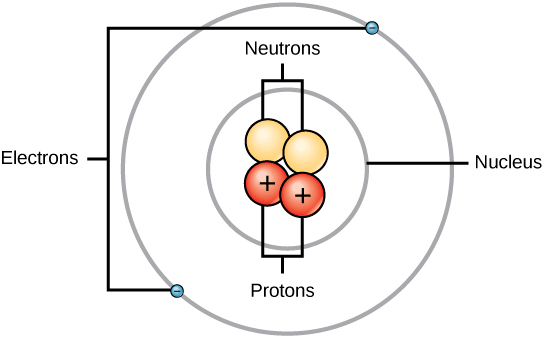
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
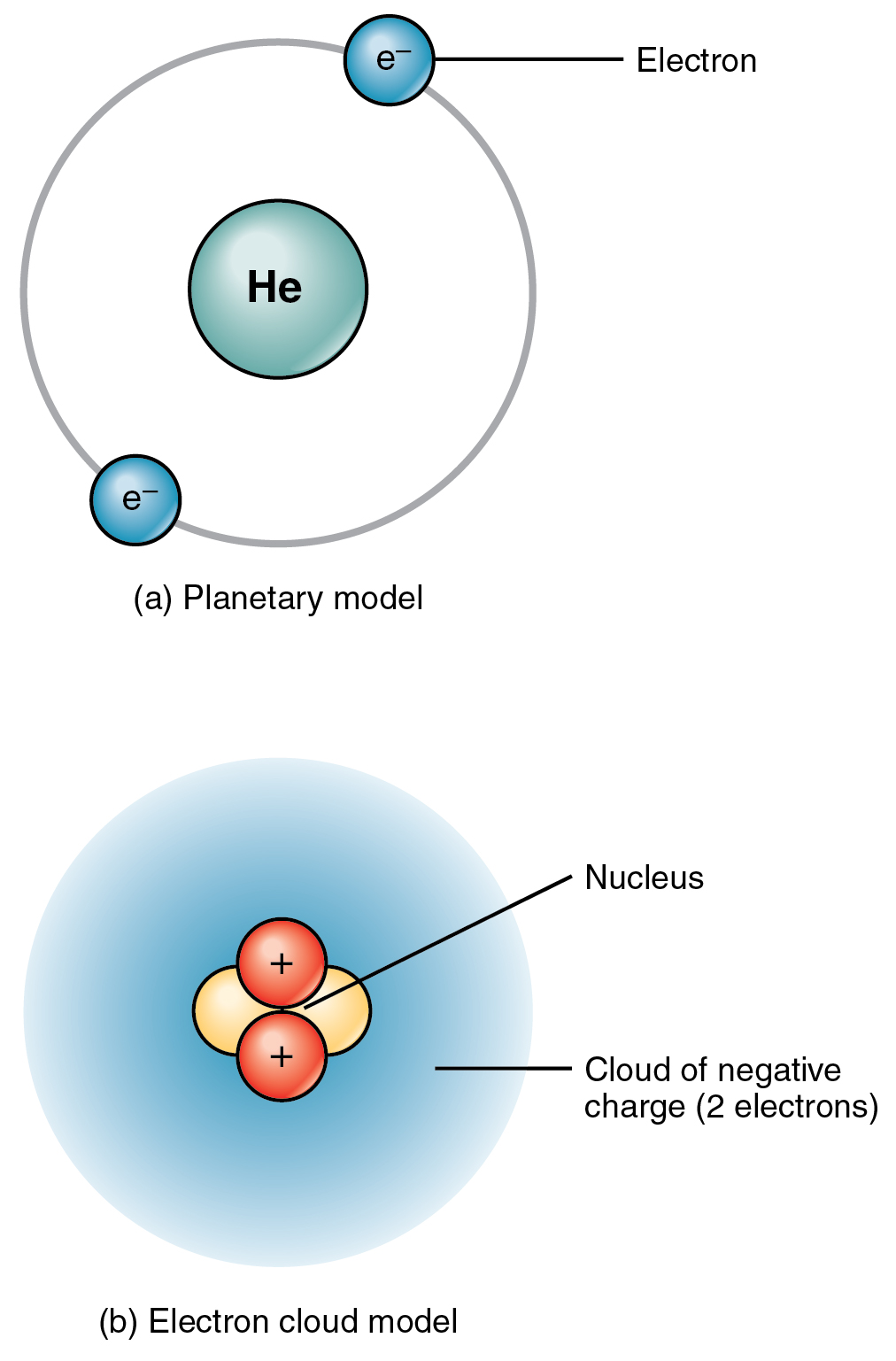
2 1 Elements And Atoms The Building Blocks Of Matter Anatomy And Physiology
Q Tbn 3aand9gctv9hbwmi7h3qtolddkv7wozp 4ythb0kgemqlvhbu8ikzanx T Usqp Cau

1 2 Atomic Structure Orbitals Chemistry Libretexts
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Basic Model Of The Atom Atomic Theory

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit

Bohr Rutherford Diagrams Lewis Dot Diagrams Eve Wongworakul Chemistry Unit

Atoms Isotopes Ions And Molecules Boundless Biology
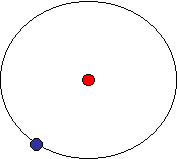
Atomic Structure
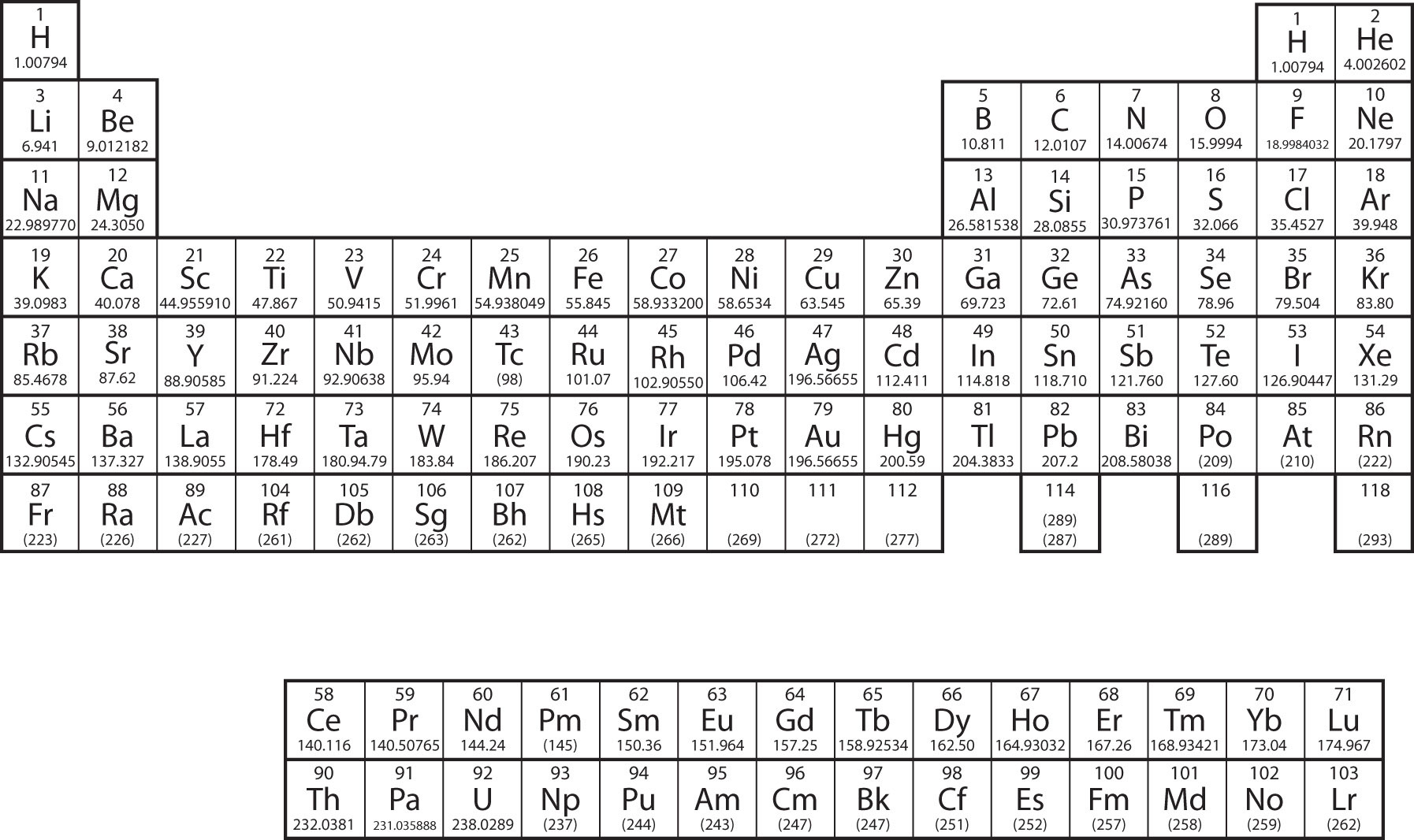
Chapter 4 Atoms Molecules And Ions Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library

Atomic Energy Levels Video Khan Academy
Q Tbn 3aand9gcryadcko5qh56frjjrqxhnao2jw1gwoufhwij 0qwsubfwr7fyj Usqp Cau

Sub Atomic Particles Atoms Siyavula
Atomic Structure
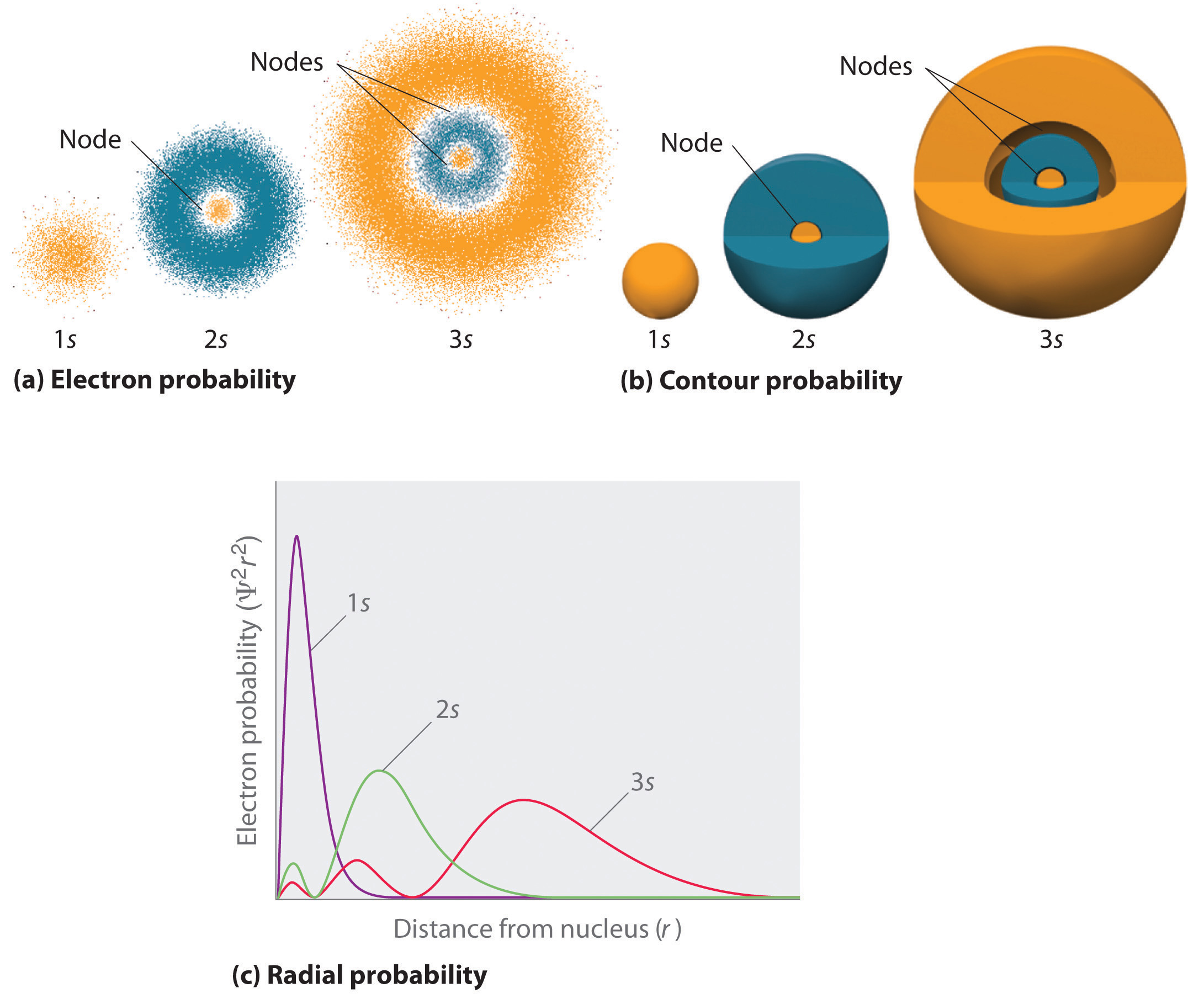
1 2 Atomic Structure Orbitals Chemistry Libretexts

Formation Of Spectral Lines Astronomy
Bohr Model Of The Atom Overview And Examples

The Development Of The Atomic Model Wired

Atomic Structure And Subatomic Particles Youtube

Rutherford Model Definition Facts Britannica

Lesson Marshmallow Molecules Betterlesson
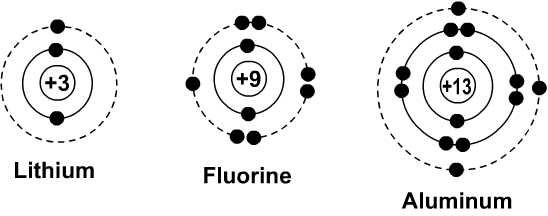
Bohr Diagrams Of Atoms And Ions Chemistry Libretexts

Atomic Structure

Rutherford Model Of The Atom Definition Diagram Video Lesson Transcript Study Com
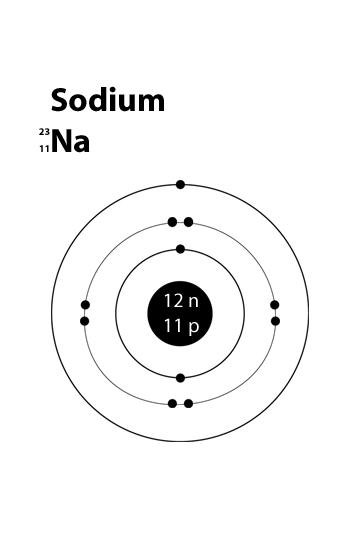
How Can I Make A Sodium Atom Model Socratic
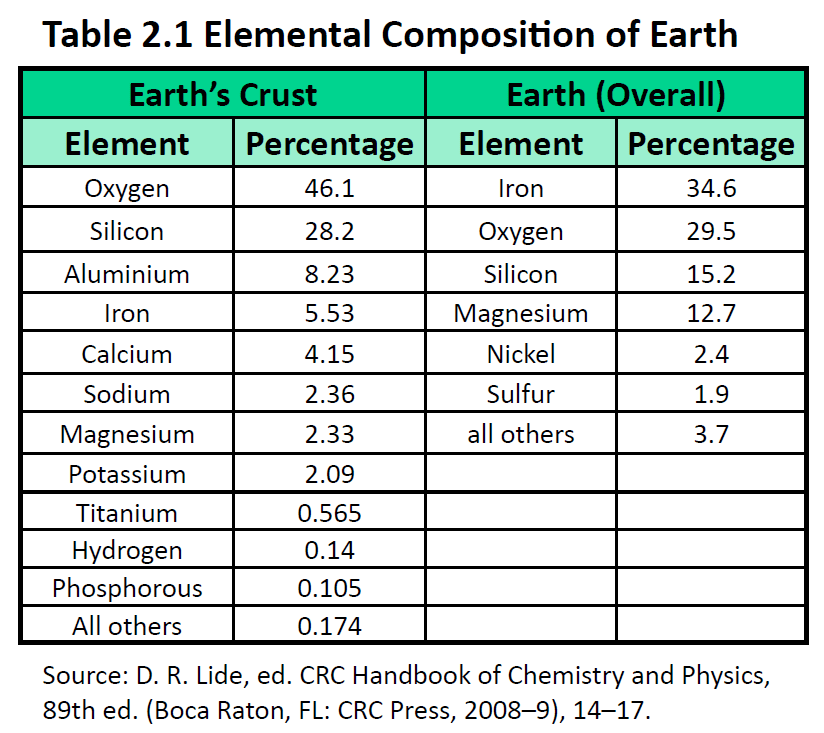
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

Dalton S Atomic Theory Article Khan Academy

Atoms What Are They What S Inside Them Explain That Stuff

Models Of The Atom The Atom Siyavula
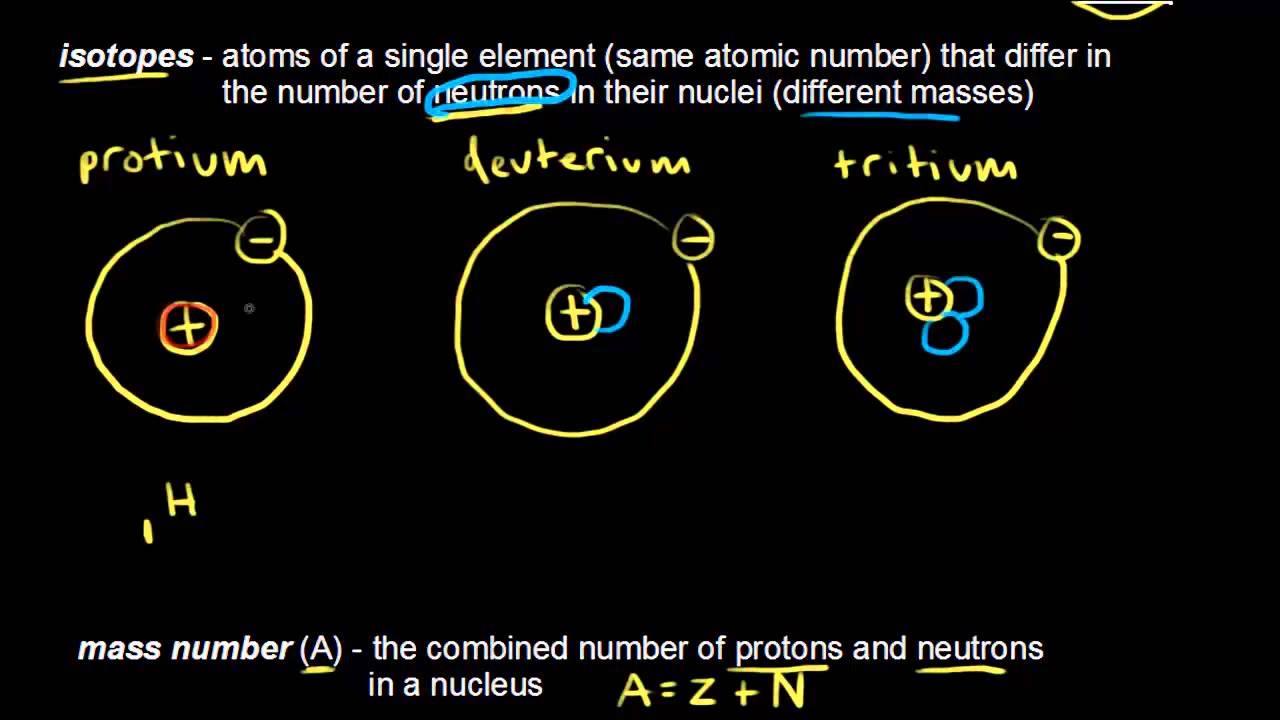
Atomic Number Mass Number And Isotopes Video Khan Academy

The Structure Of The Atom Astronomy

Atom Orbits And Energy Levels Britannica
Atomic Models Wikilectures

Atoms Isotopes Ions And Molecules Boundless Biology

What Is The Plum Pudding Atomic Model Universe Today

Models Of The Atom The Atom Siyavula

Introduction To The Atom Video Khan Academy

Bohr S Model Of Hydrogen Article Khan Academy
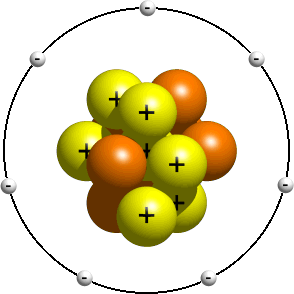
Questions And Answers How Do I Make A Model Of An Atom



